Formic Acid And Sodium Hydroxide Balanced Equation
News Leon
Mar 21, 2025 · 6 min read
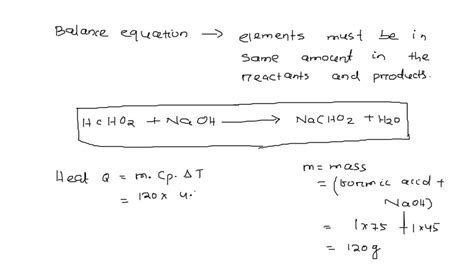
Table of Contents
Formic Acid and Sodium Hydroxide: A Deep Dive into the Balanced Equation and its Implications
Formic acid (HCOOH) and sodium hydroxide (NaOH) react in a classic acid-base neutralization reaction. Understanding this reaction, its balanced equation, and the implications of the reaction products is crucial in various scientific fields, including chemistry, environmental science, and industrial processes. This comprehensive article delves into the intricacies of this reaction, exploring its stoichiometry, applications, and safety considerations.
The Balanced Equation: A Foundation of Understanding
The reaction between formic acid and sodium hydroxide is a straightforward neutralization reaction, where the acidic hydrogen ion (H⁺) from formic acid combines with the hydroxide ion (OH⁻) from sodium hydroxide to form water (H₂O). The remaining ions, sodium (Na⁺) and formate (HCOO⁻), combine to form sodium formate (HCOONa), a salt.
The balanced chemical equation for this reaction is:
HCOOH(aq) + NaOH(aq) → HCOONa(aq) + H₂O(l)
This equation indicates that one mole of formic acid reacts with one mole of sodium hydroxide to produce one mole of sodium formate and one mole of water. The (aq) denotes that the substance is dissolved in water (aqueous solution), and (l) indicates that water is in its liquid state. The stoichiometric ratio is 1:1, meaning the reactants react in equal molar amounts.
Understanding Stoichiometry
Stoichiometry is the crucial concept that governs the quantitative relationships between reactants and products in a chemical reaction. In the case of formic acid and sodium hydroxide, the 1:1 stoichiometric ratio implies that if you have 1 mole of formic acid, you need exactly 1 mole of sodium hydroxide for complete neutralization. Any excess of either reactant will remain unreacted. This understanding is critical for performing accurate titrations and other quantitative chemical analyses.
Applications of the Formic Acid-Sodium Hydroxide Reaction
The neutralization reaction between formic acid and sodium hydroxide finds applications in various areas:
1. Acid-Base Titrations:
This reaction forms the basis of many acid-base titrations. By carefully measuring the volume of sodium hydroxide solution required to neutralize a known volume of formic acid solution, we can determine the concentration of the formic acid. This is a fundamental technique in analytical chemistry, used extensively for quality control and analysis in various industries.
2. Production of Sodium Formate:
Sodium formate, a product of this reaction, is a valuable chemical compound with various applications:
- Textile Industry: It is used as a buffering agent in textile dyeing and finishing processes.
- Food Industry: It can serve as a preservative and acidity regulator in certain food products (although its use is subject to regulations).
- Chemical Industry: It is a precursor in the synthesis of other chemicals.
- De-icing Agent: In some applications, sodium formate is used as a less corrosive de-icing agent compared to traditional salts.
The reaction provides a straightforward method for producing sodium formate. The purity and yield of the product depend on the purity of the reactants and the reaction conditions.
3. pH Control:
In various chemical processes, precise pH control is essential. The addition of sodium hydroxide to a formic acid solution can be used to carefully adjust the pH to the desired level. This is particularly relevant in applications where pH sensitivity is critical, like biochemical reactions or environmental remediation.
4. Wastewater Treatment:
Formic acid is sometimes used in industrial processes, and its neutralization with sodium hydroxide can be part of wastewater treatment to neutralize acidic waste streams before discharge. This is crucial for environmental protection and compliance with environmental regulations.
Safety Considerations and Precautions
While the reaction between formic acid and sodium hydroxide is relatively straightforward, appropriate safety measures are essential.
- Protective Equipment: Always wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a lab coat. Formic acid can cause skin irritation and burns, and sodium hydroxide is highly corrosive.
- Ventilation: Ensure adequate ventilation to prevent inhalation of fumes. The reaction can generate some heat, and proper ventilation helps to dissipate it.
- Handling Procedures: Carefully handle formic acid and sodium hydroxide solutions. Avoid spills and direct contact with skin or eyes.
- Waste Disposal: Dispose of the reaction mixture according to local regulations. The waste should be neutralized and appropriately treated before disposal.
Beyond the Basics: Exploring Reaction Kinetics and Equilibrium
While the balanced equation provides a stoichiometric overview, a deeper understanding requires consideration of reaction kinetics and equilibrium.
Reaction Kinetics:
Reaction kinetics deals with the rate at which the reaction proceeds. Factors influencing the reaction rate include:
- Concentration of Reactants: Higher concentrations of formic acid and sodium hydroxide generally lead to a faster reaction rate.
- Temperature: Increasing the temperature usually accelerates the reaction.
- Presence of Catalysts: Certain catalysts could potentially influence the reaction rate, although this is not commonly utilized in this specific reaction.
Equilibrium:
In this reaction, the equilibrium lies strongly towards the formation of sodium formate and water because the reaction is essentially irreversible under typical conditions. This means that the reaction proceeds almost completely to completion.
Applications in Different Fields: A Detailed Look
The formic acid-sodium hydroxide reaction and its product, sodium formate, have unique applications across various industries:
1. Leather Tanning:
Sodium formate plays a significant role in leather tanning. Its buffering capacity helps to maintain the optimal pH during the tanning process, ensuring high-quality leather production.
2. Pharmaceutical Industry:
Sodium formate is used as a component in some pharmaceutical formulations and as a solvent in certain drug synthesis processes. Its buffering and stabilizing properties make it suitable for these applications.
3. Agriculture:
Formic acid itself is utilized as a pesticide and preservative in certain agricultural settings. Its neutralization with sodium hydroxide might be part of its safe handling and disposal procedures.
4. Construction and Building Materials:
Sodium formate has shown potential in concrete admixtures, improving workability and reducing water consumption in concrete production.
5. Food Preservation:
Sodium formate's application in food preservation is strictly regulated. In some permitted uses, it acts as a preservative, controlling microbial growth and extending the shelf life of specific food products.
Conclusion: A Versatile Reaction with Wide-Ranging Implications
The neutralization reaction between formic acid and sodium hydroxide is a fundamental chemical reaction with far-reaching implications. From its use in precise analytical techniques to its role in the production of valuable chemicals like sodium formate, this reaction has broad applications across various scientific and industrial sectors. However, it is critical to always prioritize safety when handling these chemicals and adhere to established guidelines for their use and disposal. Understanding the balanced equation, stoichiometry, and related safety considerations is crucial for anyone working with these chemicals, ensuring efficient and safe operation while minimizing environmental impact.
Latest Posts
Latest Posts
-
How To Find Bonding And Antibonding Electrons
Mar 22, 2025
-
Which Of The Following Is The Holy Book Of Islam
Mar 22, 2025
-
A Deuterium Nucleus Contains Which Of The Following
Mar 22, 2025
-
The Process Of Making Yarn From Fibre Is Called
Mar 22, 2025
-
Name The Muscle That Subdivides The Ventral Body Cavity
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Formic Acid And Sodium Hydroxide Balanced Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
