Condensed Structural Formula For 1 2 Dibromoethane
News Leon
Mar 31, 2025 · 5 min read
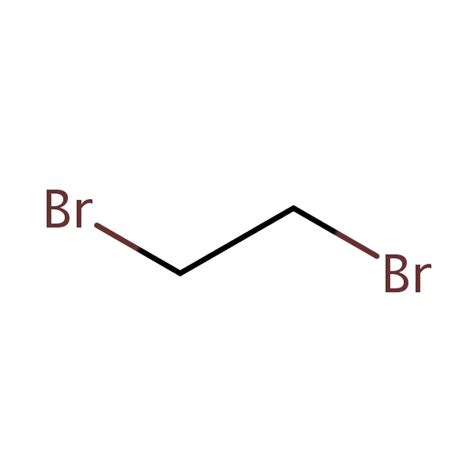
Table of Contents
Condensed Structural Formula for 1,2-Dibromoethane: A Deep Dive
1,2-Dibromoethane, also known as ethylene dibromide (EDB), is a haloalkane with a relatively simple structure, yet understanding its condensed structural formula and its implications opens doors to comprehending organic chemistry principles. This comprehensive article delves into the condensed structural formula of 1,2-dibromoethane, exploring its properties, applications (past and present), safety concerns, and its relevance in various chemical contexts.
Understanding the Condensed Structural Formula
The condensed structural formula provides a simplified representation of a molecule, omitting some bonds but retaining the essential information about atom connectivity. For 1,2-dibromoethane, the full structural formula shows each bond explicitly:
Br Br
| |
H-C-C-H
| |
H H
The condensed structural formula simplifies this representation, focusing on the arrangement of atoms:
CH₂BrCH₂Br
This concise formula clearly indicates that two carbon atoms are bonded together (implied by their adjacency), each carbon is bonded to two hydrogen atoms (indicated by the subscript '2'), and each carbon is also bonded to a bromine atom. This representation preserves the crucial information about the molecule's structure without the visual clutter of the full structural formula. The numerical prefixes (1,2) in the name "1,2-dibromoethane" explicitly define the positions of the bromine atoms on the ethane backbone.
Isomers and the Significance of Numbering
Understanding the numbering system is critical when dealing with isomers. Isomers are molecules with the same molecular formula but different structural arrangements. While 1,2-dibromoethane only has one structural isomer, the numbering system ensures unambiguous representation. For instance, if we had a molecule with different substituents on the ethane backbone, the numbering would dictate their positions and thus distinguish between different isomers. Consider a hypothetical molecule like 1-bromo-2-chloroethane: the numbering clarifies that the bromine is on carbon 1 and chlorine on carbon 2.
CH₂BrCH₂Cl (1-bromo-2-chloroethane) is distinctly different from CH₂ClCH₂Br (which would also be called 1-bromo-2-chloroethane, illustrating the inherent symmetry in this specific case).
Properties of 1,2-Dibromoethane
1,2-Dibromoethane is a colorless, oily liquid at room temperature. Key properties include:
-
Boiling Point: Relatively high compared to similar hydrocarbons due to the presence of polar C-Br bonds. These bonds lead to stronger intermolecular forces (dipole-dipole interactions).
-
Density: Denser than water, meaning it will sink if mixed with water.
-
Solubility: Slightly soluble in water but more soluble in organic solvents, reflecting the non-polar nature of the carbon-carbon and carbon-hydrogen bonds, contrasted by the relatively polar nature of the carbon-bromine bonds.
-
Reactivity: The C-Br bonds are relatively weak and susceptible to nucleophilic substitution reactions. This reactivity is a significant factor in its past applications and its environmental concerns.
Past Applications of 1,2-Dibromoethane
Historically, 1,2-dibromoethane had significant applications, primarily due to its reactivity. However, many of these applications have been phased out due to toxicity and environmental concerns. These include:
-
Lead Scavenger in Gasoline: This was its most significant past use. 1,2-Dibromoethane reacted with lead compounds in leaded gasoline, converting them into volatile lead bromide, which was then expelled from the exhaust system. The widespread use of unleaded gasoline rendered this application obsolete.
-
Soil Fumigant: Its reactivity made it effective as a soil fumigant to control nematodes and other soilborne pests. However, its toxicity to humans and the environment led to restrictions and eventual bans on its use.
-
Solvent: Its solvent properties found application in some chemical processes, but these uses have largely been replaced by safer alternatives.
Safety Concerns and Environmental Impact
The use of 1,2-dibromoethane has been severely restricted and in many cases banned due to significant health and environmental concerns:
-
Toxicity: 1,2-Dibromoethane is a known carcinogen and mutagen, posing severe health risks to humans through inhalation, skin contact, and ingestion. Exposure can lead to various health problems, including liver damage, nervous system disorders, and cancer.
-
Environmental Persistence: While it's reactive in certain contexts, it can persist in the environment for a considerable time, contaminating soil and water sources. Its bioaccumulation in the food chain also poses a significant threat to wildlife.
-
Ozone Depletion: While not as potent as chlorofluorocarbons (CFCs), it can contribute to ozone depletion.
Modern Relevance and Research
Despite its phased-out applications, 1,2-dibromoethane remains relevant in certain areas of research:
-
Synthetic Chemistry: It serves as a useful building block in the synthesis of other organic compounds, often in controlled laboratory settings with strict safety protocols.
-
Environmental Remediation: Research continues into its breakdown mechanisms and potential for bioremediation—using microorganisms to degrade it in contaminated environments.
-
Toxicology and Risk Assessment: Understanding its toxicity mechanisms is crucial for developing safer alternatives and mitigating environmental risks associated with related compounds.
Comparing 1,2-Dibromoethane to other Haloalkanes
Understanding the structure and properties of 1,2-dibromoethane allows for comparison with other haloalkanes:
-
Dichloroethanes: Compounds like 1,2-dichloroethane have similar structures but differ in their reactivity and toxicity. The difference in halogen atoms (chlorine instead of bromine) impacts the strength of the carbon-halogen bonds and their environmental fate.
-
Bromoalkanes in general: 1,2-dibromoethane serves as a representative example of the bromoalkane family. Variations in the length of the carbon chain and the number and positions of bromine atoms lead to different properties and applications.
-
Other Dihalides: Comparing it with other dihalides, such as 1,1-dibromoethane, highlights the impact of isomerism on chemical and physical properties. 1,1-dibromoethane, for example, will exhibit different reactivity and physical properties due to its differing structural configuration.
Conclusion
The condensed structural formula, CH₂BrCH₂Br, provides a concise and essential representation of 1,2-dibromoethane's structure. While its past uses have been largely curtailed due to safety and environmental concerns, understanding its properties and reactivity remains crucial for several reasons. Its role as a case study in organic chemistry, its significance in toxicological studies, and its potential for future research in bioremediation highlight its continued relevance within scientific disciplines. The lessons learned from its use underscore the importance of careful evaluation of the environmental and health implications of chemical compounds before widespread application. Continued research and development of safer alternatives are vital in minimizing environmental damage and protecting human health.
Latest Posts
Latest Posts
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
-
Lewis Dot Structure For Magnesium Chloride
Apr 01, 2025
-
A Group Of Related Records Is Called A Table
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Condensed Structural Formula For 1 2 Dibromoethane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
