Complete The Following Table Using The Periodic Table
News Leon
Mar 28, 2025 · 6 min read
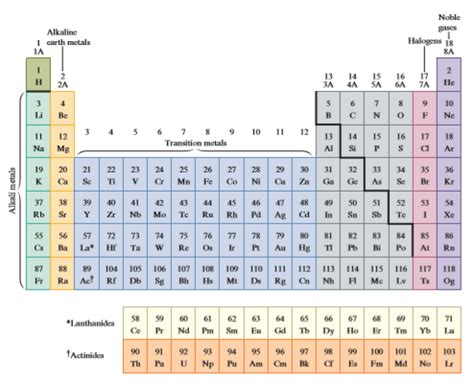
Table of Contents
Completing the Periodic Table: A Comprehensive Guide
The periodic table is a cornerstone of chemistry, organizing elements based on their atomic number, electron configuration, and recurring chemical properties. Mastering its use is crucial for understanding chemical reactions, predicting compound behavior, and delving deeper into the fascinating world of atoms and molecules. This article will guide you through the process of completing a periodic table, explaining the logic behind its structure and providing practical exercises. We'll explore how to deduce element properties from their position, emphasizing the importance of understanding trends and patterns.
Understanding the Structure of the Periodic Table
Before we begin completing a table, let's review its fundamental structure. The table is arranged in rows (periods) and columns (groups or families).
Periods: Horizontal Rows
Each period represents an energy level or electron shell. Elements within the same period have electrons filling the same principal energy level. The number of elements in a period varies, reflecting the increasing complexity of electron configurations. For example, Period 1 contains only two elements (Hydrogen and Helium) because it only has one energy level (n=1), which can hold a maximum of two electrons.
Groups: Vertical Columns
Elements in the same group share similar chemical properties because they have the same number of valence electrons – the electrons in the outermost shell. These valence electrons are primarily responsible for the element's reactivity and bonding characteristics. Groups are numbered from 1 to 18.
- Group 1 (Alkali Metals): Highly reactive metals with one valence electron.
- Group 2 (Alkaline Earth Metals): Reactive metals with two valence electrons.
- Groups 3-12 (Transition Metals): Metals with variable oxidation states and complex chemistry.
- Group 17 (Halogens): Highly reactive nonmetals with seven valence electrons.
- Group 18 (Noble Gases): Inert gases with a full valence shell (eight electrons, except for Helium with two).
Blocks: Regions of the Table
The periodic table is further subdivided into blocks based on the type of orbital the valence electrons occupy:
- s-block: Groups 1 and 2 (alkali and alkaline earth metals). Valence electrons occupy the s orbital.
- p-block: Groups 13-18. Valence electrons occupy the p orbital.
- d-block: Groups 3-12 (transition metals). Valence electrons occupy the d orbital.
- f-block: Lanthanides and Actinides (placed separately at the bottom). Valence electrons occupy the f orbital.
Completing a Blank Periodic Table: A Step-by-Step Approach
Let's assume you have a blank periodic table with only the atomic numbers provided. How do you populate it with element symbols, names, and other properties?
Step 1: Identifying the Elements:
The atomic number uniquely identifies each element. This number corresponds to the number of protons in the nucleus and dictates the element's position on the table. Begin by writing the element symbol (e.g., H for Hydrogen, He for Helium) in its appropriate location based on its atomic number.
Step 2: Determining Electron Configuration:
Knowing the atomic number, you can determine the electron configuration. This follows the Aufbau principle (filling orbitals in order of increasing energy) and Hund's rule (filling orbitals singly before pairing). The electron configuration determines the element's group (valence electrons) and period (energy level). For example, Oxygen (atomic number 8) has an electron configuration of 1s²2s²2p⁴. This tells us it's in Period 2 (highest energy level is 2) and Group 16 (six valence electrons – 2s²2p⁴).
Step 3: Assigning Element Names and Symbols:
Once you’ve placed the element symbols based on atomic numbers, write the corresponding element names below. For instance, if you have the symbol 'O', write 'Oxygen' underneath.
Step 4: Identifying Elemental Properties:
Based on the element's group and period, you can predict many properties:
- Metallic Character: Generally increases down a group and decreases across a period (from left to right).
- Reactivity: Alkali metals (Group 1) are highly reactive, while noble gases (Group 18) are inert. Halogens (Group 17) are also highly reactive nonmetals.
- Electronegativity: Tendency of an atom to attract electrons in a chemical bond. It increases across a period and decreases down a group.
- Ionization Energy: Energy required to remove an electron from an atom. It increases across a period and decreases down a group.
- Atomic Radius: Size of an atom. It increases down a group and decreases across a period.
Step 5: Adding Additional Information (Optional):
Depending on the level of detail required, you might include additional information like:
- Atomic Mass: The average mass of an atom of an element, considering the isotopes.
- Oxidation States: Possible charges an atom can have when it forms a compound.
- Melting and Boiling Points: Physical properties indicating the strength of interatomic forces.
- Density: Mass per unit volume.
Practical Exercises and Examples
Let’s complete a small section of the periodic table as an illustrative example. Let's focus on elements with atomic numbers 11 to 17.
| Atomic Number | Element Symbol | Element Name | Group | Period | Valence Electrons |
|---|---|---|---|---|---|
| 11 | Na | Sodium | 1 | 3 | 1 |
| 12 | Mg | Magnesium | 2 | 3 | 2 |
| 13 | Al | Aluminium | 13 | 3 | 3 |
| 14 | Si | Silicon | 14 | 3 | 4 |
| 15 | P | Phosphorus | 15 | 3 | 5 |
| 16 | S | Sulfur | 16 | 3 | 6 |
| 17 | Cl | Chlorine | 17 | 3 | 7 |
Notice the trends: As we move from left to right across Period 3, the number of valence electrons increases, leading to changes in chemical properties. Sodium and Magnesium are metals, while Phosphorus, Sulfur, and Chlorine are nonmetals. Silicon is a metalloid, exhibiting properties of both metals and nonmetals.
Advanced Considerations: Isotopes and Atomic Mass
The atomic mass listed in the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. Isotopes are atoms of the same element with different numbers of neutrons. This means they have the same atomic number (number of protons) but different mass numbers (protons + neutrons). The atomic mass reflects the relative abundance of each isotope.
Conclusion: Mastering the Periodic Table
The periodic table is an invaluable tool for any chemistry student or enthusiast. Understanding its structure and the relationships between elements' positions and properties is fundamental. Through systematic practice and a focus on trends and patterns, one can confidently complete a periodic table and use it to predict and understand the behavior of various elements and their compounds. Remember to practice regularly, using various exercises and examples to solidify your understanding. By following the steps outlined above and engaging with numerous practice problems, you'll not only improve your ability to complete the periodic table but also significantly enhance your comprehension of fundamental chemistry concepts.
Latest Posts
Latest Posts
-
A Person Who Study History Is Called
Mar 31, 2025
-
Is Calcium Oxide Ionic Or Covalent
Mar 31, 2025
-
What Is Not True Regarding Antibiotics
Mar 31, 2025
-
Balanced Equation For Copper And Nitric Acid
Mar 31, 2025
-
Passwords Passphrases And Pins Are Examples Of Which Security Term
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Complete The Following Table Using The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
