Calculate The Molarity Of Each Of The Following Solutions
News Leon
Mar 21, 2025 · 5 min read
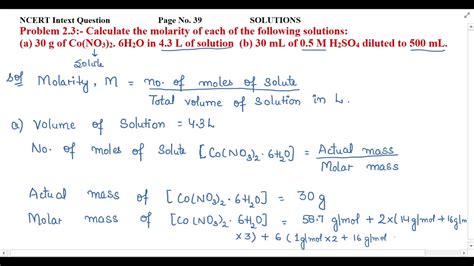
Table of Contents
Calculate the Molarity of Each of the Following Solutions: A Comprehensive Guide
Molarity, a cornerstone concept in chemistry, quantifies the concentration of a solution. Understanding how to calculate molarity is crucial for various applications, from preparing solutions in a laboratory to comprehending chemical reactions. This comprehensive guide will walk you through the process of calculating molarity for different scenarios, offering practical examples and helpful tips to solidify your understanding.
Understanding Molarity
Molarity (M) is defined as the number of moles of solute per liter of solution. The formula is:
Molarity (M) = Moles of solute / Liters of solution
To calculate molarity, you'll need to determine both the moles of solute and the volume of the solution. Let's break down each component:
1. Moles of Solute
The "solute" is the substance being dissolved. To find the moles of solute, you'll typically use the molar mass of the solute. The molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). You can find molar masses on the periodic table for elements or calculate them for compounds by summing the atomic masses of all constituent atoms.
Formula: Moles = Mass (g) / Molar Mass (g/mol)
2. Liters of Solution
The "solution" is the homogeneous mixture of solute and solvent. The volume of the solution must be expressed in liters (L) for the molarity calculation. If the volume is given in milliliters (mL), remember to convert it to liters by dividing by 1000 (1 L = 1000 mL).
Calculating Molarity: Step-by-Step Examples
Now, let's delve into practical examples to illustrate the calculation of molarity.
Example 1: Simple Molarity Calculation
-
Problem: Calculate the molarity of a solution prepared by dissolving 5.85 g of NaCl (sodium chloride) in enough water to make 250 mL of solution.
-
Step 1: Find the molar mass of NaCl.
- Na (sodium) has a molar mass of approximately 22.99 g/mol.
- Cl (chlorine) has a molar mass of approximately 35.45 g/mol.
- Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
-
Step 2: Calculate the moles of NaCl.
- Moles of NaCl = Mass (g) / Molar Mass (g/mol) = 5.85 g / 58.44 g/mol ≈ 0.100 moles
-
Step 3: Convert the volume to liters.
- Volume = 250 mL / 1000 mL/L = 0.250 L
-
Step 4: Calculate the molarity.
- Molarity (M) = Moles of solute / Liters of solution = 0.100 moles / 0.250 L = 0.400 M
-
Answer: The molarity of the NaCl solution is 0.400 M.
Example 2: Molarity Calculation with a More Complex Compound
-
Problem: Determine the molarity of a solution containing 20.0 g of glucose (C₆H₁₂O₆) in 500 mL of solution.
-
Step 1: Find the molar mass of glucose (C₆H₁₂O₆).
- C (carbon): 12.01 g/mol × 6 = 72.06 g/mol
- H (hydrogen): 1.01 g/mol × 12 = 12.12 g/mol
- O (oxygen): 16.00 g/mol × 6 = 96.00 g/mol
- Molar mass of glucose = 72.06 g/mol + 12.12 g/mol + 96.00 g/mol = 180.18 g/mol
-
Step 2: Calculate the moles of glucose.
- Moles of glucose = 20.0 g / 180.18 g/mol ≈ 0.111 moles
-
Step 3: Convert the volume to liters.
- Volume = 500 mL / 1000 mL/L = 0.500 L
-
Step 4: Calculate the molarity.
- Molarity (M) = 0.111 moles / 0.500 L = 0.222 M
-
Answer: The molarity of the glucose solution is 0.222 M.
Example 3: Dilutions and Molarity
Often, you need to dilute a concentrated solution to a lower concentration. This involves using the dilution formula:
M₁V₁ = M₂V₂
where:
-
M₁ = initial molarity
-
V₁ = initial volume
-
M₂ = final molarity
-
V₂ = final volume
-
Problem: You have 100 mL of a 2.0 M solution of HCl. You want to dilute it to a 0.5 M solution. What will be the final volume?
-
Step 1: Identify your knowns.
- M₁ = 2.0 M
- V₁ = 100 mL = 0.100 L
- M₂ = 0.5 M
- V₂ = ?
-
Step 2: Use the dilution formula.
- (2.0 M)(0.100 L) = (0.5 M)(V₂)
- V₂ = (2.0 M)(0.100 L) / 0.5 M = 0.400 L = 400 mL
-
Answer: You need to dilute the HCl solution to a final volume of 400 mL to achieve a 0.5 M concentration.
Advanced Molarity Calculations and Considerations
Beyond the basic calculations, several factors can influence molarity calculations:
1. Temperature Effects
Molarity is temperature-dependent. As temperature increases, the volume of the solution may change, affecting the molarity. For precise work, temperature control is essential.
2. Ionic Compounds and Dissociation
When calculating the molarity of solutions containing ionic compounds, consider the dissociation of the compound into its ions in solution. For instance, 1 M NaCl solution contains 1 M Na⁺ ions and 1 M Cl⁻ ions.
3. Molarity of Mixed Solutions
Calculating the molarity of a mixture of solutions requires considering the total number of moles of solute and the total volume of the solution. This involves adding the moles of each solute and dividing by the total volume.
4. Density Considerations
In some cases, the density of the solution may be known and can be used to determine the volume. The relationship between mass, volume, and density is:
Density = Mass / Volume
Practical Applications of Molarity Calculations
Molarity calculations are fundamental across numerous chemical disciplines:
- Titrations: Determining the concentration of an unknown solution by reacting it with a solution of known molarity.
- Stoichiometry: Calculating the amounts of reactants and products in chemical reactions.
- Spectrophotometry: Relating the absorbance of a solution to its concentration, often using molar absorptivity.
- Pharmaceutical Preparations: Precisely preparing medications and ensuring accurate dosages.
- Environmental Monitoring: Determining the concentrations of pollutants in water and air samples.
Conclusion
Calculating molarity is a critical skill for anyone working with solutions in chemistry and related fields. This comprehensive guide has provided a thorough explanation of the concept, along with step-by-step examples to solidify your understanding. Remember to pay close attention to units, and consider the advanced factors that can influence molarity calculations in more complex scenarios. Mastering molarity calculations empowers you to tackle various chemical problems with precision and confidence. Keep practicing and refining your skills to become proficient in this fundamental aspect of chemistry.
Latest Posts
Latest Posts
-
How To Center Text In Python
Mar 22, 2025
-
An Equilibrium Mixture Of Pcl5 Pcl3 And Cl2
Mar 22, 2025
-
Which Statement Best Describes The Colonial Attitude Before The 1760s
Mar 22, 2025
-
Which Of The Following Can Exist As A Meso Isomer
Mar 22, 2025
-
Double Displacement Reaction Examples In Real Life
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Molarity Of Each Of The Following Solutions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
