Which Of The Following Can Exist As A Meso Isomer
News Leon
Mar 22, 2025 · 5 min read
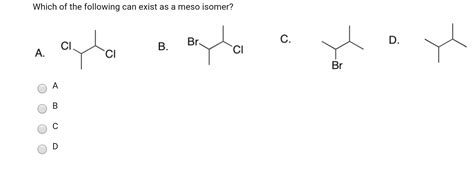
Table of Contents
Which of the Following Can Exist as a Meso Isomer? A Deep Dive into Stereochemistry
Understanding meso isomers is crucial for anyone studying organic chemistry. These unique compounds possess chiral centers yet exhibit an overall achiral nature due to internal symmetry. This seemingly paradoxical characteristic makes them a fascinating subject of study and a frequent source of confusion for students. This comprehensive guide will explore the concept of meso isomers, detailing their properties, identifying criteria for their existence, and providing numerous examples to solidify your understanding. We'll delve into how to distinguish them from other stereoisomers and explain their significance in various chemical contexts.
What are Meso Compounds?
A meso compound is a molecule that contains multiple stereocenters (chiral centers) but possesses an internal plane of symmetry. This symmetry renders the molecule achiral, even though it contains chiral carbons. It's crucial to remember that the presence of chiral centers alone does not guarantee chirality. The presence of an internal plane of symmetry cancels out the optical activity that would otherwise be expected. As a result, meso compounds are optically inactive, meaning they don't rotate plane-polarized light.
Key Characteristics of Meso Isomers:
- Multiple Stereocenters: Meso compounds must have at least two stereocenters. A single chiral center automatically results in a chiral molecule.
- Internal Plane of Symmetry: The defining characteristic of a meso compound is the presence of an internal plane of symmetry. This plane divides the molecule into two halves that are mirror images of each other.
- Optically Inactive: Despite possessing chiral centers, meso compounds are optically inactive because the rotation of plane-polarized light caused by one half of the molecule is exactly canceled out by the rotation caused by the other half.
- Diastereomers of Chiral Molecules: Meso compounds are diastereomers (stereoisomers that are not mirror images) of other chiral molecules with the same connectivity. They are not enantiomers (mirror image isomers).
Distinguishing Meso Compounds from Other Stereoisomers:
It's vital to be able to distinguish meso compounds from other stereoisomers, particularly enantiomers and diastereomers.
- Enantiomers: Enantiomers are non-superimposable mirror images. They have identical physical properties except for their interaction with plane-polarized light (they rotate it in opposite directions). Meso compounds are not enantiomers because they are achiral.
- Diastereomers: Diastereomers are stereoisomers that are not mirror images. Meso compounds are diastereomers of the chiral molecules with the same connectivity. They differ in their physical and chemical properties.
Identifying Potential Meso Isomers:
To determine whether a molecule can exist as a meso isomer, carefully examine its structure for the presence of:
-
At Least Two Stereocenters: The molecule must possess at least two chiral carbons.
-
Internal Plane of Symmetry: This is the most critical aspect. Imagine a plane slicing through the molecule. If the molecule can be divided into two identical halves that are mirror images of each other, then an internal plane of symmetry exists, indicating the possibility of a meso isomer.
Let's look at some examples to illustrate this concept:
Example 1: Tartaric Acid
Tartaric acid is a classic example used to explain meso compounds. It has two chiral centers. One stereoisomer of tartaric acid is chiral, while another is a meso compound. The meso-tartaric acid possesses an internal plane of symmetry, whereas the chiral forms do not.
Example 2: 2,3-Dibromobutane
2,3-Dibromobutane has two chiral centers. It exists as three stereoisomers: two enantiomers and one meso compound. The meso isomer exhibits an internal plane of symmetry.
Example 3: 1,2-Dibromocyclohexane
Certain substituted cyclohexanes can also exhibit meso isomerism. For 1,2-dibromocyclohexane, the cis isomer possesses an internal plane of symmetry and is therefore a meso compound. The trans isomer, on the other hand, is chiral.
Example 4: Molecules with More Than Two Stereocenters
Meso isomerism is not limited to molecules with only two chiral centers. Compounds with more stereocenters can also exist as meso isomers if they possess internal symmetry. Identifying the plane of symmetry becomes more challenging, but the principle remains the same.
Practical Applications and Significance:
Understanding meso isomers has significant implications in various areas:
- Organic Synthesis: The ability to predict and synthesize meso compounds is crucial for organic chemists. It influences reaction pathways and product selectivity.
- Pharmaceutical Chemistry: Meso isomers can have different biological activities compared to their chiral counterparts. This is especially relevant in drug design and development. One isomer might be therapeutically active while another is inactive or even toxic.
- Material Science: Meso compounds can exhibit unique physical properties that are exploited in the design of materials with specific characteristics.
Advanced Considerations:
- Conformational Isomers: It's important to note that conformational analysis plays a role in determining whether a meso isomer exists. Certain conformations might possess a plane of symmetry while others do not. The presence of a meso form requires a conformation that maintains the plane of symmetry.
- Multiple Planes of Symmetry: Some molecules possess multiple planes of symmetry. This further emphasizes their achiral nature.
Conclusion:
Meso compounds are fascinating examples of molecules that defy initial expectations. While possessing chiral centers, their internal symmetry renders them achiral and optically inactive. Understanding the criteria for their existence, their relationship to other stereoisomers, and their practical applications is vital for a comprehensive grasp of stereochemistry. By carefully examining the molecular structure for the presence of at least two stereocenters and an internal plane of symmetry, one can accurately identify potential meso isomers and appreciate their unique role in the world of chemistry. This knowledge is critical for success in organic chemistry and various related fields. Remember to practice identifying meso isomers using diverse examples to strengthen your understanding and ability to quickly and accurately classify these unique molecules. The more you practice, the more intuitive the process will become. Don't hesitate to explore additional resources and work through practice problems to solidify your grasp of this essential concept.
Latest Posts
Latest Posts
-
Which Of The Following Is A Non Essential Fatty Acid
Mar 23, 2025
-
Which Of The Following Is Not An Intangible Asset
Mar 23, 2025
-
How Many Seconds In 365 Days
Mar 23, 2025
-
An Electron Has An Initial Velocity Of
Mar 23, 2025
-
How Many Corners On A Cube
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Can Exist As A Meso Isomer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
