Boiling Of Water Is A Physical Change
News Leon
Mar 23, 2025 · 5 min read
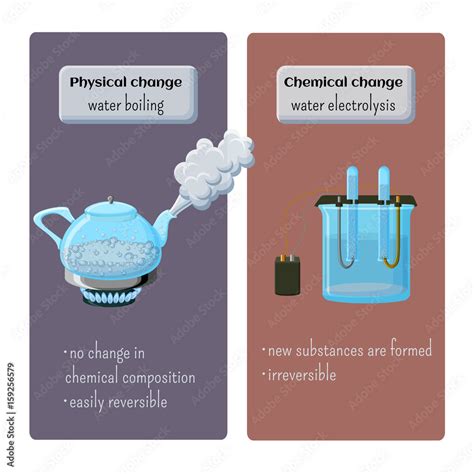
Table of Contents
Boiling Water: A Deep Dive into Physical Change
Boiling water is a quintessential example of a physical change, a transformation that alters the form or appearance of matter without changing its chemical composition. While seemingly simple, the process reveals fundamental principles of physics and chemistry, demonstrating the interplay between temperature, pressure, and the states of matter. This detailed exploration will delve into the specifics of boiling water, clarifying its physical nature and differentiating it from chemical changes.
Understanding Physical Changes vs. Chemical Changes
Before focusing on the boiling of water, it's crucial to understand the distinction between physical and chemical changes. Physical changes are reversible; the original substance can be recovered. Examples include melting ice, freezing water, dissolving sugar in water, and – the focus of this article – boiling water. The water molecules remain water molecules throughout the entire process.
In contrast, chemical changes (or chemical reactions) are irreversible. New substances with different chemical properties are formed. Burning wood, rusting iron, and cooking an egg are all examples of chemical changes. The original materials are transformed into entirely new substances.
The Science Behind Boiling Water
The boiling of water is a phase transition, specifically a change from liquid water to gaseous water (water vapor or steam). This transformation is driven by the kinetic energy of water molecules.
Molecular Movement and Temperature
Water molecules are constantly in motion, vibrating and colliding with each other. Temperature is a measure of the average kinetic energy of these molecules. As the temperature increases, the molecules move faster and faster. At a certain point – 100°C (212°F) at standard atmospheric pressure – the kinetic energy of the molecules overcomes the intermolecular forces holding them together in the liquid state.
Breaking Intermolecular Bonds
These intermolecular forces, primarily hydrogen bonds, are relatively strong in water, accounting for its high boiling point compared to other similar molecules. At the boiling point, enough energy is supplied to break a significant number of these bonds, allowing molecules to escape the liquid phase and transition into the gaseous phase.
The Role of Pressure
Atmospheric pressure plays a crucial role in determining the boiling point. Atmospheric pressure is the force exerted by the weight of the air above a given point. This pressure pushes down on the surface of the water, hindering the escape of water molecules. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, water boils at a higher temperature. This principle is exploited in pressure cookers, where increased pressure allows food to cook at higher temperatures, resulting in faster cooking times.
Evaporation vs. Boiling
While both evaporation and boiling involve the transition of water from liquid to gas, there are key differences. Evaporation is a surface phenomenon; it occurs at the surface of a liquid at any temperature. The molecules with the highest kinetic energy escape from the surface, gradually reducing the overall temperature of the liquid.
Boiling, on the other hand, occurs throughout the entire liquid. It is a much more rapid process, characterized by the formation of bubbles of water vapor within the liquid itself. These bubbles rise to the surface and burst, releasing water vapor into the atmosphere. Boiling only occurs at the boiling point for a given pressure.
Evidence of Boiling Water as a Physical Change
Several observations support the classification of boiling water as a physical change:
-
Reversible Process: If you collect the steam produced by boiling water and allow it to cool, it condenses back into liquid water. This demonstrates the reversibility characteristic of physical changes. The chemical composition of the water remains unchanged throughout the entire process.
-
No New Substance Formed: Boiling water doesn't produce any new chemical substances. The steam produced is still H₂O, just in a different physical state. There's no chemical reaction involved.
-
Retention of Chemical Properties: The water molecules in the steam retain the same chemical properties as the liquid water. For instance, the water will still quench your thirst, just as liquid water does. This chemical property persists regardless of the physical state.
-
Separation Techniques: The process of boiling water can be reversed using simple separation techniques, like condensation. This further emphasizes the absence of any chemical transformation.
Misconceptions about Boiling Water
Several common misconceptions exist concerning boiling water:
-
Boiling purifies water: While boiling effectively kills many harmful microorganisms, it doesn't remove all impurities. Dissolved minerals and some chemicals remain in the water even after boiling.
-
All impurities evaporate with the steam: Most dissolved substances do not evaporate with steam. Only volatile compounds will do so.
-
Boiling changes the taste of water: While boiling may alter the taste slightly due to the removal of some volatile compounds, it doesn't fundamentally change the chemical composition of the water itself. Any changes in taste are subtle and linked to the removal of volatile elements, not a chemical alteration.
Applications and Implications of Boiling Water
The process of boiling water has numerous applications across various fields:
-
Cooking: Boiling is a fundamental cooking method used to prepare countless dishes.
-
Sterilization: Boiling water is an effective method for sterilizing utensils and equipment, killing harmful bacteria and viruses.
-
Water Purification: Boiling water is a simple and effective way to improve the safety of drinking water, although it does not remove all contaminants.
-
Industrial Processes: Boiling is employed in various industrial processes, such as steam generation for power plants and chemical processing.
-
Scientific Experiments: Boiling water is used in numerous scientific experiments to demonstrate concepts related to heat transfer, phase transitions, and thermodynamics.
Conclusion: The Undeniable Physical Nature of Boiling
The process of boiling water serves as a clear and concise example of a physical change. It involves a transition between different physical states of matter – liquid to gas – without altering the chemical composition of the substance. The reversibility of the process, the absence of new substance formation, and the retention of chemical properties all point towards the physical nature of this transformation. While seemingly simple, the boiling of water offers profound insights into the molecular behavior of matter and the fundamental principles of thermodynamics. Understanding this seemingly simple process provides a solid foundation for comprehending more complex chemical and physical phenomena. The thorough understanding and application of this knowledge are crucial in diverse fields, emphasizing the importance of studying even the most commonplace processes in great depth.
Latest Posts
Latest Posts
-
A Projectile Is Fired Horizontally From A Gun
Mar 25, 2025
-
Why Europe Is Called The Peninsula Of Peninsulas
Mar 25, 2025
-
Which Of The Following Is A Mineralocorticoid
Mar 25, 2025
-
Which Of The Following Is Not A Benefit Of Insurance
Mar 25, 2025
-
What Happens To The Plant Cell In A Hypertonic Solution
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Boiling Of Water Is A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
