What Happens To The Plant Cell In A Hypertonic Solution
News Leon
Mar 25, 2025 · 6 min read
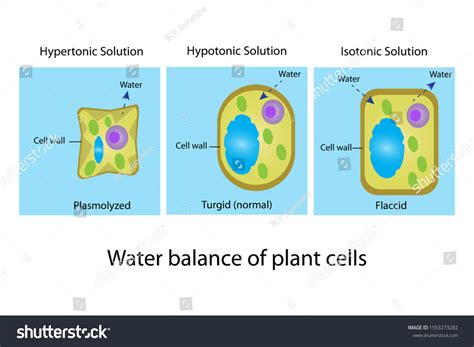
Table of Contents
What Happens to a Plant Cell in a Hypertonic Solution? A Deep Dive into Plasmolysis
Plant cells, unlike animal cells, possess a rigid cell wall surrounding the delicate cell membrane. This structural difference significantly impacts how they respond to changes in their environment, particularly when exposed to solutions of varying solute concentrations. Understanding the effects of hypertonic solutions on plant cells is crucial for comprehending plant physiology, agriculture, and even certain medical applications. This comprehensive article will explore the intricacies of plasmolysis, the process by which plant cells shrink in a hypertonic solution, detailing the mechanisms involved and the consequences for the plant.
Understanding Osmosis and Tonicity
Before diving into the specifics of plant cell behavior in a hypertonic solution, it’s vital to establish a solid understanding of osmosis and tonicity.
Osmosis is the passive movement of water across a selectively permeable membrane from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration). This movement continues until equilibrium is reached, or the water potential is equal on both sides of the membrane.
Tonicity describes the relative concentration of solutes in two solutions separated by a selectively permeable membrane. There are three main types of tonicity:
- Isotonic: The solute concentration is equal on both sides of the membrane. There is no net movement of water.
- Hypotonic: The solute concentration is lower outside the cell than inside the cell. Water moves into the cell.
- Hypertonic: The solute concentration is higher outside the cell than inside the cell. Water moves out of the cell.
Plasmolysis: The Shrinking of Plant Cells in a Hypertonic Solution
When a plant cell is placed in a hypertonic solution, water moves out of the cell via osmosis. This is because the water potential inside the cell is higher than the water potential outside the cell. The loss of water causes the cell's central vacuole, a large fluid-filled sac that occupies a significant portion of the cell's volume, to shrink. This shrinkage pulls the cell membrane away from the cell wall, a process known as plasmolysis.
Stages of Plasmolysis
Plasmolysis doesn't happen instantaneously. It occurs in stages:
- Incipient Plasmolysis: This is the initial stage where the plasma membrane just begins to pull away from the cell wall at the corners. The cell still retains some turgor pressure, but it's significantly reduced.
- Plasmolysis: As water continues to leave the cell, the plasma membrane detaches further from the cell wall, forming distinct gaps. The cell loses its turgor pressure completely, becoming flaccid.
- Extreme Plasmolysis (Cytorrhysis): In extreme cases, the protoplast (the cell's contents excluding the cell wall) completely shrinks and rounds up, pulling away significantly from the cell wall. This is irreversible, leading to cell death.
The Role of the Cell Wall
The presence of a cell wall is what distinguishes the response of a plant cell to a hypertonic solution from that of an animal cell. The cell wall provides structural support and prevents the cell from bursting in a hypotonic solution. However, in a hypertonic solution, the cell wall cannot prevent the loss of water and the consequent shrinkage of the protoplast. The cell wall remains intact, but the plasma membrane pulls away from it.
Factors Influencing Plasmolysis
Several factors influence the rate and extent of plasmolysis:
- Concentration of the Hypertonic Solution: The greater the difference in solute concentration between the solution and the cell's cytoplasm, the faster and more pronounced the plasmolysis will be.
- Type of Solute: Different solutes can have different effects on the cell membrane's permeability, influencing the rate of water movement.
- Temperature: Higher temperatures generally increase the rate of osmosis, potentially accelerating plasmolysis.
- Cell Type: Different plant cells have varying levels of water permeability and may respond differently to the same hypertonic solution.
- Plant Species: Different plant species exhibit different tolerances to water stress and thus varying degrees of plasmolysis resistance.
Consequences of Plasmolysis
Plasmolysis is not just a laboratory observation; it has significant implications for plant survival and function:
- Loss of Turgor Pressure: The most immediate consequence is the loss of turgor pressure, the pressure exerted by the cell contents against the cell wall. Turgor pressure is essential for maintaining cell shape, plant rigidity, and the overall structural integrity of the plant. Loss of turgor pressure leads to wilting and drooping.
- Impaired Cellular Processes: Plasmolysis disrupts various cellular processes, including nutrient uptake, photosynthesis, and respiration. The shrinkage of the cytoplasm can hinder the movement of organelles and the efficiency of metabolic reactions.
- Reduced Growth: The inability to maintain turgor pressure restricts cell expansion, resulting in stunted growth and reduced overall plant size.
- Death of Cells: If plasmolysis is severe and prolonged, it can lead to irreversible damage and the eventual death of the plant cells.
Deplasmolysis: Reversing Plasmolysis
Under certain conditions, plasmolysis can be reversed. If the plant cell is transferred to a hypotonic solution or a solution with a lower solute concentration than the cell's cytoplasm, water will move back into the cell via osmosis. This process, known as deplasmolysis, involves the re-establishment of turgor pressure and the return of the plasma membrane to its original position against the cell wall. The speed of deplasmolysis depends on factors such as the initial extent of plasmolysis, the concentration of the hypotonic solution, and the plant cell's physiological state.
Practical Applications and Significance
Understanding plasmolysis has practical applications in various fields:
- Agriculture: Farmers need to understand how different soil conditions and watering practices affect the water potential in plant cells and the potential for plasmolysis. Optimal irrigation management can prevent excessive water loss and wilting.
- Food Preservation: Plasmolysis can be used to preserve food by creating a hypertonic environment that inhibits microbial growth. Salting and sugaring are examples of traditional methods that use this principle.
- Medicine: Understanding osmosis and plasmolysis is important in designing solutions for intravenous fluids and other medical applications, ensuring that cells don't lose or gain excessive amounts of water.
Conclusion: A Dynamic Process with Far-Reaching Effects
Plasmolysis, the shrinkage of plant cells in a hypertonic solution, is a fundamental process with significant consequences for plant survival and function. It highlights the intricate interplay between water movement, cell structure, and overall plant physiology. Understanding the mechanisms of plasmolysis and its implications is crucial for optimizing plant growth, developing effective preservation techniques, and advancing various fields of science and technology. From the wilting of a garden plant to the preservation of food, the principles governing plasmolysis are widely applicable and essential to comprehend the complex world of plant biology. Further research continually expands our understanding of this vital process and its applications in diverse areas.
Latest Posts
Related Post
Thank you for visiting our website which covers about What Happens To The Plant Cell In A Hypertonic Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
