An Epitope Associated With Which Part Of An Antibody
News Leon
Apr 05, 2025 · 7 min read
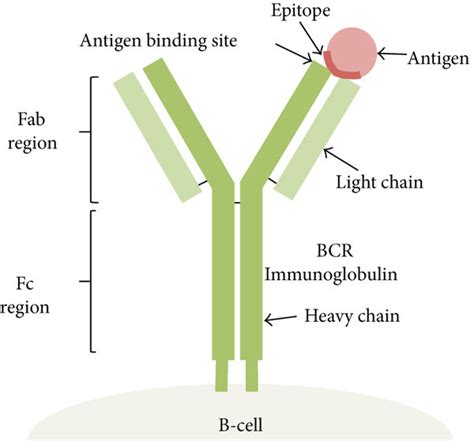
Table of Contents
The Intricate Dance: Epitope Location and Antibody Binding
Antibodies, the body's specialized defense force, are Y-shaped proteins that recognize and neutralize specific foreign invaders, like bacteria and viruses. This recognition hinges on a precise interaction between a specific region on the antibody, the paratope, and a corresponding region on the antigen, known as the epitope. Understanding the relationship between epitope location and antibody binding is crucial for developing effective vaccines, diagnostics, and therapeutic antibodies. This article delves deep into the intricacies of this interaction, exploring the various types of epitopes, the influence of epitope location on antibody binding, and the implications for immunology and biotechnology.
Understanding the Players: Antibodies and Antigens
Before exploring the epitope-antibody interaction, let's briefly review the key players.
Antibodies (Immunoglobulins): The Body's Defense
Antibodies, also known as immunoglobulins (Ig), are glycoproteins produced by plasma B cells. Their structure is crucial to their function. The Y-shape consists of two identical heavy chains and two identical light chains, linked by disulfide bonds. Each chain contains variable (V) and constant (C) regions. The variable regions, located at the tips of the Y's arms (also known as Fab regions, fragment antigen-binding), are highly diverse and responsible for antigen recognition. Within the variable regions are hypervariable regions or complementarity-determining regions (CDRs), which form the paratope, the antibody's antigen-binding site. The constant regions determine the antibody's isotype (IgM, IgG, IgA, IgE, IgD) and mediate effector functions such as complement activation and interaction with immune cells.
Antigens: The Targets of Antibody Recognition
Antigens are any substance that can elicit an immune response. They can be proteins, polysaccharides, lipids, or nucleic acids. The specific region on the antigen that binds to the antibody's paratope is the epitope. An antigen can possess multiple epitopes, each capable of binding to a different antibody. The location of the epitope on the antigen significantly influences the antibody's ability to bind and neutralize it.
Epitope Classification: Location Matters
Epitopes can be broadly classified based on their location and structural characteristics:
1. Linear Epitopes (Sequential Epitopes):
- Definition: These epitopes are formed by a continuous sequence of amino acids in a protein's primary structure. They are accessible to the antibody even when the protein is folded into its native conformation.
- Recognition: Antibody recognition of linear epitopes is relatively straightforward, relying on the specific amino acid sequence.
- Impact of protein folding: Protein folding minimally affects the accessibility of linear epitopes.
- Example: A short stretch of amino acids exposed on the surface of a protein.
2. Conformational Epitopes (Discontinuous Epitopes):
- Definition: These epitopes are formed by amino acids that are not contiguous in the primary sequence but are brought together in the protein's three-dimensional structure. They are dependent on the protein's tertiary or quaternary structure.
- Recognition: Antibody recognition requires the protein to be in its native folded state. Denaturation of the protein will typically abolish the binding.
- Impact of protein folding: Protein folding is essential for the formation and presentation of conformational epitopes.
- Example: Amino acids from different regions of a protein that come together to form a specific surface patch.
3. Neo-Epitopes:
- Definition: These epitopes are formed only after post-translational modifications, such as glycosylation, phosphorylation, or proteolytic cleavage, alter the protein structure.
- Recognition: Antibody recognition is specifically directed towards the modified amino acid sequence or newly formed structure.
- Impact of protein modification: Post-translational modifications are crucial to the formation of neo-epitopes and the presentation to antibodies.
- Example: A glycosylation site exposed upon the glycosylation of a protein.
4. Cryptic Epitopes:
- Definition: These epitopes are hidden within the protein's interior or masked by other structures and are usually inaccessible to antibodies.
- Recognition: Antibody binding only occurs under specific conditions, such as protein denaturation or conformational changes.
- Impact of protein conformation: Protein conformation actively shields these epitopes from recognition.
- Example: Amino acid sequence buried inside a protein's hydrophobic core.
The Influence of Epitope Location on Antibody Binding
The location of an epitope profoundly impacts several aspects of antibody binding:
1. Accessibility:
- Surface exposure: Epitopes located on the protein's surface are more accessible to antibodies, leading to stronger and faster binding.
- Steric hindrance: Epitopes buried within the protein's interior or sterically hindered by other molecules may not be readily accessible.
2. Affinity:
- Strength of binding: The affinity of an antibody for an epitope depends on various factors, including the number and type of interactions between the paratope and epitope.
- Shape complementarity: The better the shape complementarity between the paratope and epitope, the stronger the binding affinity.
3. Specificity:
- Unique features: The unique structural characteristics of an epitope define the specificity of antibody binding.
- Cross-reactivity: Some antibodies may exhibit cross-reactivity, binding to similar epitopes on different antigens.
4. Immunogenicity:
- Immune response: Epitopes located on the protein's surface tend to elicit stronger immune responses, leading to higher antibody production.
- Conformational changes: Conformational changes that expose cryptic epitopes might induce a new immune response.
Epitope Mapping: Unveiling the Binding Sites
To understand the specifics of antibody-epitope interactions, techniques are needed for epitope mapping: determining the precise location and structural characteristics of the epitope recognized by an antibody. Several methods exist, including:
- X-ray crystallography and NMR spectroscopy: High-resolution techniques to visualize the three-dimensional structure of the antibody-antigen complex.
- Peptide scanning: Systematic testing of overlapping peptides derived from an antigen to identify the minimum sequence required for antibody binding.
- Alanine scanning mutagenesis: Systematic replacement of amino acid residues within an antigen with alanine to identify key residues involved in antibody binding.
- Hydrogen-deuterium exchange mass spectrometry (HDX-MS): Measures changes in hydrogen-deuterium exchange rates upon antibody binding to identify the regions of the antigen involved in the interaction.
- Computational methods: In silico predictions and modeling of potential epitope locations based on sequence and structural information.
Implications for Immunology and Biotechnology
Understanding the relationship between epitope location and antibody binding has significant implications for various fields:
1. Vaccine Design:
- Selecting immunogenic epitopes: Identifying and using highly immunogenic epitopes in vaccine development ensures the generation of a robust immune response.
- Targeting specific epitopes: Vaccines can be designed to target specific epitopes on pathogens to elicit neutralizing antibodies.
2. Diagnostic Development:
- Developing specific assays: Identifying specific epitopes allows for the development of sensitive and specific diagnostic tests for various diseases.
- Improving detection sensitivity: Antibody-based assays targeting highly accessible epitopes will show improved sensitivity.
3. Therapeutic Antibody Development:
- Engineering high-affinity antibodies: Knowledge of epitope location is valuable in engineering antibodies with enhanced affinity and specificity for therapeutic applications.
- Targeting specific epitopes on cancer cells: The identification of tumor-associated antigens and their epitopes is crucial for developing targeted therapies.
4. Understanding Autoimmune Diseases:
- Identifying autoantigens and epitopes: Mapping epitopes recognized by autoantibodies helps in understanding the mechanisms of autoimmune diseases.
- Developing therapies: Knowledge of autoantigen epitopes is important for developing therapies such as epitope-based vaccines or immunomodulatory strategies.
Conclusion
The interplay between epitope location and antibody binding is a fundamental aspect of the immune system's ability to recognize and neutralize foreign invaders. The accessibility, affinity, specificity, and immunogenicity of an epitope are all influenced by its location and structural characteristics. Understanding these intricate interactions is crucial for advancing our knowledge of immunology and for developing innovative vaccines, diagnostics, and therapeutics. Further research into epitope mapping and the mechanisms of antibody-epitope recognition will undoubtedly continue to yield valuable insights into the complex world of immunology and its clinical applications. The continuous improvement of epitope mapping techniques and the development of new bioinformatics tools will only increase our ability to leverage this understanding for the development of effective and innovative immunotherapeutic strategies. The future of immunology and its related fields relies heavily on the continued unraveling of the intricacies of antibody-epitope interactions.
Latest Posts
Latest Posts
-
What Chemical Element Has The Highest Electron Affinity
Apr 05, 2025
-
Why Does The Dna Double Helix Have A Uniform Diameter
Apr 05, 2025
-
In Guinea Pigs The Allele For Short Hair Is Dominant
Apr 05, 2025
-
An Electrically Charged Atom Or Group Of Atoms
Apr 05, 2025
-
What Phylum Does The Crayfish Belong To
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about An Epitope Associated With Which Part Of An Antibody . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
