An Alkaline Earth Metal In Period 3
News Leon
Mar 14, 2025 · 6 min read
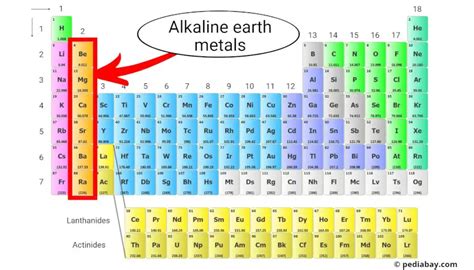
Table of Contents
Magnesium: The Unsung Alkaline Earth Metal of Period 3
Magnesium, a shining star in the periodic table's third period, often plays second fiddle to its more flamboyant neighbors. While not as reactive as its alkali metal counterpart, sodium, or as ubiquitous as the ubiquitous transition metals, magnesium holds a pivotal role in various biological and industrial processes. This in-depth exploration dives into the fascinating world of magnesium, uncovering its properties, reactions, applications, and ecological significance.
Unveiling Magnesium's Properties: A Closer Look
Magnesium (Mg), atomic number 12, sits comfortably in Group 2 of the periodic table, the alkaline earth metals. This placement dictates its characteristic properties:
Electronic Configuration and Reactivity:
Magnesium's electronic configuration, [Ne] 3s², reveals its two valence electrons readily available for chemical bonding. This configuration drives its reactivity, although less vigorous than alkali metals due to the higher effective nuclear charge. This means that magnesium's two outer electrons are more strongly attracted to the nucleus than a sodium atom's single outer electron, hence requiring more energy to remove.
Physical Properties:
- Appearance: A silvery-white, lightweight metal with a slightly dull surface due to rapid oxidation in air.
- Density: Relatively low density (1.74 g/cm³), making it a strong contender for lightweight applications.
- Melting and Boiling Points: Relatively low melting point (650°C) and boiling point (1090°C) compared to many other metals, reflecting its relatively weak metallic bonding.
- Hardness and Strength: Magnesium possesses a moderate level of hardness and tensile strength, making it suitable for various structural applications.
- Electrical and Thermal Conductivity: Mg exhibits good electrical and thermal conductivity, contributing to its uses in electrical components and heat exchangers.
Chemical Properties:
Magnesium's reactivity stems from its tendency to lose its two valence electrons, forming the Mg²⁺ ion. This property dictates its behavior in various chemical reactions:
- Reaction with Oxygen: Magnesium readily reacts with oxygen in the air, forming magnesium oxide (MgO), a white powder commonly known as magnesia. This reaction is highly exothermic, producing intense heat and light, often used in flares and fireworks. The reaction equation is: 2Mg(s) + O₂(g) → 2MgO(s)
- Reaction with Acids: Magnesium reacts vigorously with most acids, liberating hydrogen gas. The reaction with hydrochloric acid (HCl) is a classic example: Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
- Reaction with Water: While magnesium's reaction with cold water is relatively slow, it reacts more readily with hot water or steam, producing magnesium hydroxide and hydrogen gas.
- Reaction with Nitrogen: At high temperatures, magnesium reacts with nitrogen gas to form magnesium nitride (Mg₃N₂).
Magnesium's Diverse Applications: From Aerospace to Medicine
Magnesium's unique blend of properties translates into a wide spectrum of applications across various industries:
Aerospace and Automotive Industries:
Magnesium's low density and relatively high strength make it an invaluable material for aerospace and automotive components. Its use in lightweight alloys significantly improves fuel efficiency in vehicles and reduces the weight of aircraft, leading to better performance and reduced fuel consumption. Magnesium alloys find application in:
- Aircraft parts: Engine casings, instrument panels, and other structural components.
- Automotive parts: Wheels, dashboards, and body panels.
Biomedical Applications:
Magnesium's biocompatibility and essential role in biological processes have established it as a crucial material in biomedical applications. These include:
- Biodegradable implants: Magnesium alloys are being developed as biodegradable implants, eliminating the need for secondary surgery to remove the implant after healing. The magnesium slowly degrades in the body, releasing magnesium ions which are beneficial to the body.
- Drug delivery systems: Magnesium-based materials are being explored as drug delivery systems, providing controlled release of therapeutic agents.
Other Applications:
Magnesium also finds applications in various other fields:
- Metallurgy: Magnesium is used as an alloying agent in the production of aluminum alloys and other metal mixtures, enhancing their strength and other properties.
- Photography: Magnesium powder or ribbon is used in flash photography due to its bright combustion.
- Pyrotechnics: Magnesium contributes to the dazzling brilliance of fireworks.
- Chemical Industry: Magnesium compounds find applications as catalysts, reducing agents, and drying agents.
- Electronics: Magnesium is used in batteries, providing energy in certain battery types.
Magnesium's Biological Significance: An Essential Element of Life
Magnesium is not merely an industrial workhorse; it's a vital element for all living organisms. Its presence in the body is essential for numerous biological processes:
Enzyme Activation:
Magnesium acts as a cofactor for numerous enzymes, playing a crucial role in metabolism and various biochemical reactions. It activates enzymes involved in:
- DNA and RNA synthesis: Crucial processes for cell growth and reproduction.
- Protein synthesis: The production of proteins that perform a wide range of cellular functions.
- Energy production: The production of ATP (adenosine triphosphate), the cell's energy currency.
Muscle Contraction:
Magnesium plays a vital role in muscle contraction and relaxation. Its presence is essential for proper muscle function, preventing muscle cramps and spasms.
Nerve Function:
Magnesium helps regulate nerve function, influencing nerve impulses and ensuring proper communication between nerve cells.
Blood Sugar Control:
Magnesium plays a role in blood sugar control, helping to regulate insulin sensitivity and prevent the development of type 2 diabetes.
Blood Pressure Regulation:
Magnesium's presence contributes to blood pressure regulation, potentially mitigating the risk of hypertension.
A deficiency in magnesium can lead to several health problems, emphasizing its importance in maintaining overall health and well-being. Symptoms of magnesium deficiency include muscle cramps, fatigue, and weakness.
Environmental Considerations: Sustainable Use of Magnesium
While magnesium is relatively abundant in the Earth's crust, its extraction and processing require careful consideration of environmental impacts. Sustainable practices are crucial to minimize these impacts:
Energy Consumption:
Magnesium production is energy-intensive, requiring significant amounts of electricity. Reducing energy consumption through process optimization is vital for minimizing the carbon footprint.
Waste Management:
Magnesium processing generates waste products that require proper management to prevent environmental pollution. Implementing responsible waste management practices, such as recycling and waste reduction strategies, is crucial.
Recycling:
Recycling magnesium is environmentally beneficial, reducing the demand for new magnesium extraction and minimizing associated environmental impacts. Developing efficient magnesium recycling processes is essential for sustainability.
Future Prospects: Research and Development in Magnesium
Research and development in magnesium continue to explore new avenues for utilizing its unique properties. Future areas of focus include:
Advanced Magnesium Alloys:
Developing advanced magnesium alloys with enhanced properties, such as higher strength, improved corrosion resistance, and tailored biocompatibility, is a significant area of ongoing research.
Biodegradable Implants:
Research is focused on optimizing the biodegradability and biocompatibility of magnesium-based implants, ensuring safe and effective degradation within the body.
Sustainable Production Methods:
Efforts are underway to develop more sustainable magnesium production methods, minimizing energy consumption and environmental impacts.
Novel Applications:
Exploring novel applications for magnesium in various fields, such as energy storage, hydrogen production, and water treatment, offers exciting possibilities for the future.
Conclusion: The Enduring Importance of Magnesium
Magnesium, the unassuming alkaline earth metal of period 3, holds a significant place in both industrial and biological spheres. Its lightweight nature, reactivity, and biocompatibility provide a diverse array of applications. From aerospace engineering to biomedical implants and its essential role in human health, magnesium plays a multifaceted role in modern society. As research continues to unlock new applications and optimize sustainable production methods, magnesium's importance is only set to grow in the years to come. Its future is bright, reflecting its multifaceted contributions to a world increasingly focused on sustainability and innovation.
Latest Posts
Latest Posts
-
How Many Valence Electrons Does Kr Have
Mar 14, 2025
-
A Flywheel With A Diameter Of 1 20m Is Rotating
Mar 14, 2025
-
Is Al Oh 3 Soluble In Water
Mar 14, 2025
-
The Number Of Protons In An Atom Is Called
Mar 14, 2025
-
Which Of The Following Cells Is Phagocytic
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about An Alkaline Earth Metal In Period 3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
