How Many Valence Electrons Does Kr Have
News Leon
Mar 14, 2025 · 5 min read
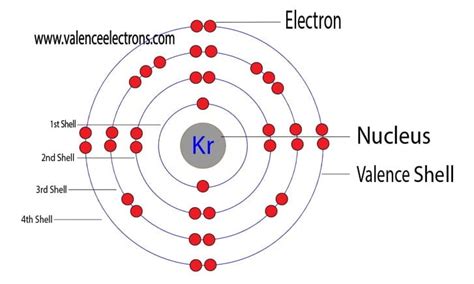
Table of Contents
How Many Valence Electrons Does Kr Have? A Deep Dive into Krypton's Electronic Structure
Krypton (Kr), a noble gas residing in Group 18 of the periodic table, is renowned for its chemical inertness. This inertness stems directly from its electronic configuration, specifically the number of valence electrons it possesses. Understanding this number is key to grasping Krypton's properties and its behavior in various contexts. This article will delve deeply into the question: how many valence electrons does Kr have? We'll explore the underlying principles of electron configuration, the significance of valence electrons, and the implications of Krypton's electronic structure.
Understanding Electron Configuration and Valence Electrons
Before we determine Krypton's valence electrons, let's establish a foundational understanding of electron configuration. The electron configuration of an atom describes how electrons are distributed among the various energy levels and sublevels within the atom. These energy levels are often represented by principal quantum numbers (n = 1, 2, 3, etc.), while sublevels are designated by letters (s, p, d, f). Each sublevel can hold a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons
- p sublevel: Holds a maximum of 6 electrons
- d sublevel: Holds a maximum of 10 electrons
- f sublevel: Holds a maximum of 14 electrons
Valence electrons are the electrons located in the outermost energy level (highest principal quantum number) of an atom. These electrons are crucial because they are primarily involved in chemical bonding and determine an element's chemical reactivity. Atoms tend to interact with each other in ways that result in a stable, filled outer electron shell, often resembling the electron configuration of the nearest noble gas.
Determining Krypton's Electron Configuration
Krypton (Kr) has an atomic number of 36, meaning it possesses 36 protons and 36 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy:
1s², 2s², 2p⁶, 3s², 3p⁶, 4s², 3d¹⁰, 4p⁶
This configuration can also be written in a shorthand notation, using the noble gas preceding Krypton (Argon, Ar) as a starting point:
[Ar] 4s² 3d¹⁰ 4p⁶
Identifying Krypton's Valence Electrons
Now, let's pinpoint Krypton's valence electrons. Looking at the full electron configuration or the shorthand notation, we observe that the outermost energy level is the fourth energy level (n=4). The electrons in this level are the 4s² and 4p⁶ electrons. Therefore, Krypton has a total of 8 valence electrons (2 + 6 = 8).
This is a key characteristic of noble gases. They typically possess a full outer electron shell with eight valence electrons (except for Helium, which has two). This complete outer shell is exceptionally stable, contributing to their chemical inertness.
The Significance of Krypton's Eight Valence Electrons
Krypton's eight valence electrons are the cornerstone of its chemical behavior:
-
Inertness: The complete octet of valence electrons makes Krypton extremely unreactive. It rarely forms chemical bonds with other elements under normal conditions. This is because it doesn't need to gain, lose, or share electrons to achieve a stable electron configuration.
-
Noble Gas Configuration: The stable octet represents a noble gas configuration, a highly desirable state for atoms. Many atoms undergo chemical reactions to achieve this stable configuration.
-
Applications: While Krypton itself is largely unreactive, its unique electronic structure allows it to be utilized in specific applications, leveraging its inertness and other properties. For example, it's used in some lighting applications (e.g., Krypton-filled lamps) and in lasers.
Exceptions and Further Considerations
While the octet rule (the tendency for atoms to have eight valence electrons) is a useful guideline, there are exceptions. Some atoms can achieve stability with less than or more than eight valence electrons. However, for Krypton, the octet rule perfectly explains its chemical inertness.
Krypton's Role in Advanced Applications
Despite its inertness, Krypton plays a role in various specialized applications:
-
Lighting: Krypton gas is used in some types of lighting, including high-intensity discharge lamps and fluorescent lights. Its unique spectral lines contribute to the light emitted.
-
Lasers: Krypton is used in specific types of lasers, creating monochromatic light with precise wavelengths. These lasers find applications in various scientific and industrial fields.
-
Medicine: Certain Krypton isotopes are employed in medical imaging and treatment techniques, harnessing their radioactive properties.
-
Other Uses: Krypton also finds niche applications in other fields, such as high-energy physics research and analytical chemistry.
Conclusion: Understanding Krypton's Valence Electrons
In conclusion, Krypton (Kr) possesses eight valence electrons. This complete octet of valence electrons is responsible for its exceptional chemical inertness and its unique properties, making it valuable in specialized applications despite its lack of reactivity. Understanding the electron configuration and the significance of valence electrons is crucial for comprehending the behavior of all elements, including the noble gases like Krypton. The stable electronic structure of Krypton is a fundamental aspect of its chemistry and its role in various scientific and technological advancements. Further study of its properties and applications continues to reveal new insights into this intriguing element.
Keywords:
Krypton, valence electrons, electron configuration, noble gas, chemical inertness, octet rule, atomic number, periodic table, chemical reactivity, electron shell, Aufbau principle, Krypton applications, lighting, lasers, medicine, noble gas configuration.
Related Keywords:
Electron orbitals, quantum numbers, chemical bonding, periodic trends, ionization energy, electronegativity, atomic structure, spectral lines, isotopes, radioactive isotopes, high-intensity discharge lamps, fluorescent lamps.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Renewable Resource
Mar 15, 2025
-
How Many Faces Are There On A Standard Dice
Mar 15, 2025
-
Which Of The Following Is Not A Function Of Skin
Mar 15, 2025
-
Which Phase Is The Longest In The Cell Cycle
Mar 15, 2025
-
Lines Of Symmetry On A Square
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Kr Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
