A Negatively Charged Ion Is Called A
News Leon
Mar 17, 2025 · 6 min read
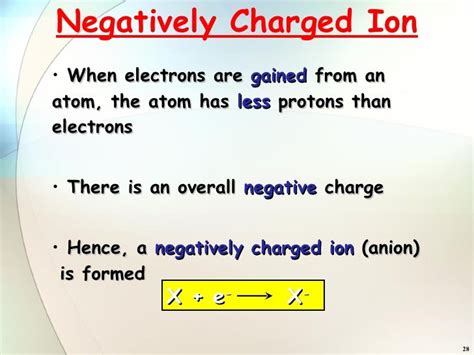
Table of Contents
A Negatively Charged Ion is Called an Anion: A Deep Dive into Ionic Chemistry
A negatively charged ion is called an anion. Understanding anions is crucial to grasping fundamental concepts in chemistry, physics, and biology. This comprehensive guide explores the fascinating world of anions, delving into their formation, properties, nomenclature, and significant roles in various fields. We'll also examine related concepts like cations, ionic bonds, and the broader implications of ionic interactions.
Understanding Ions: The Building Blocks of Ionic Compounds
Before diving into the specifics of anions, let's establish a foundational understanding of ions. Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net electrical charge. This charge imbalance is what distinguishes ions from neutral atoms or molecules.
Cations: Positively Charged Ions
The counterpart to anions are cations, which carry a positive charge. Cations are formed when an atom loses one or more electrons, leaving it with more protons than electrons. Metals, with their relatively low electronegativity, tend to form cations. Examples include sodium ions (Na⁺), calcium ions (Ca²⁺), and potassium ions (K⁺).
Anions: Negatively Charged Ions
As mentioned earlier, a negatively charged ion is specifically termed an anion. Anions are created when an atom gains one or more electrons, resulting in more electrons than protons. Nonmetals, with their higher electronegativity, typically form anions. Examples include chloride ions (Cl⁻), oxide ions (O²⁻), and sulfide ions (S²⁻).
The Formation of Anions: A Closer Look at Electron Gain
The formation of an anion is driven by the atom's desire to achieve a stable electron configuration, often resembling that of a noble gas. Noble gases have a full outermost electron shell (valence shell), making them exceptionally stable. Atoms gain electrons to fill their valence shell and achieve this stable state.
Electronegativity and Anion Formation
Electronegativity plays a vital role in anion formation. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. Atoms with high electronegativity have a strong tendency to attract electrons and form anions. Elements like chlorine, oxygen, and fluorine are highly electronegative and readily form anions.
Ionic Bonds: The Attraction Between Opposites
The electrostatic attraction between a cation and an anion forms an ionic bond. This strong attractive force holds the ions together in a crystal lattice structure, forming an ionic compound. The overall charge of an ionic compound is neutral, meaning the positive charges of the cations are balanced by the negative charges of the anions. Table salt (NaCl), for example, is an ionic compound formed by the attraction between sodium cations (Na⁺) and chloride anions (Cl⁻).
Naming Anions: A Systematic Approach
The naming of anions follows a systematic approach based on the element's name and its charge.
Monatomic Anions: Simple Anions from Single Atoms
Monatomic anions are derived from single atoms that have gained electrons. These are named by adding the suffix "-ide" to the root name of the element. For instance:
- Chloride (Cl⁻): Derived from chlorine
- Oxide (O²⁻): Derived from oxygen
- Sulfide (S²⁻): Derived from sulfur
- Nitride (N³⁻): Derived from nitrogen
- Fluoride (F⁻): Derived from fluorine
- Bromide (Br⁻): Derived from bromine
- Iodide (I⁻): Derived from iodine
Polyatomic Anions: Complex Anions from Multiple Atoms
Polyatomic anions are composed of multiple atoms covalently bonded together and carrying a net negative charge. Their naming is more complex and often involves memorizing common names:
- Hydroxide (OH⁻): Found in bases and many organic compounds.
- Nitrate (NO₃⁻): A crucial component in fertilizers and explosives.
- Sulfate (SO₄²⁻): Used in various industrial processes and found in some minerals.
- Phosphate (PO₄³⁻): Essential for biological processes like energy transfer.
- Carbonate (CO₃²⁻): Found in limestone and other carbonates.
- Bicarbonate (HCO₃⁻): A crucial component of blood's buffering system.
- Permanganate (MnO₄⁻): A strong oxidizing agent used in various chemical reactions and as a disinfectant.
- Chromate (CrO₄²⁻) and Dichromate (Cr₂O₇²⁻): Used in various industrial applications and chemical analyses.
The Significance of Anions in Various Fields
Anions play critical roles in various aspects of our world, impacting several scientific disciplines:
Biology and Biochemistry
Anions are essential for numerous biological processes. For example:
- Phosphate (PO₄³⁻): Crucial for energy transfer (ATP), DNA structure, and cell signaling.
- Chloride (Cl⁻): Maintains fluid balance, nerve impulse transmission, and gastric acid production.
- Bicarbonate (HCO₃⁻): Acts as a buffer, regulating blood pH.
- Hydroxide (OH⁻): Involved in various enzymatic reactions.
Imbalances in anion levels can lead to various health issues, highlighting their critical role in maintaining homeostasis.
Chemistry and Materials Science
Anions are fundamental building blocks of many materials:
- Ionic Compounds: Many salts, minerals, and ceramics are composed of ionic compounds involving anions.
- Electrolytes: Anions are crucial components of electrolytes, which conduct electricity in solution.
- Catalysis: Certain anions act as catalysts in various chemical reactions.
Environmental Science
Anions impact environmental processes significantly:
- Nitrate (NO₃⁻): A major component of water pollution from fertilizers, leading to eutrophication.
- Sulfate (SO₄²⁻): Contributes to acid rain, damaging ecosystems.
- Fluoride (F⁻): Used in water fluoridation to prevent tooth decay, but excess fluoride can have adverse effects.
Understanding the behavior and environmental impact of anions is crucial for maintaining ecological balance.
Medicine and Pharmacology
Many drugs and pharmaceutical compounds are based on anions or contain anions as part of their structure. Understanding how anions interact with biological systems is crucial in drug development and therapy. Some examples include:
- Anionic surfactants: Used in many pharmaceutical formulations for their solubilizing properties.
- Phosphate buffers: Used in various pharmaceutical preparations to maintain stability.
Advanced Concepts and Further Exploration
The world of anions extends beyond the basic principles discussed here. Further exploration might involve:
- Spectroscopy: Techniques like infrared and Raman spectroscopy can be used to identify and characterize different anions.
- Crystallography: X-ray diffraction studies reveal the arrangement of anions and cations in crystal lattices.
- Electrochemistry: The behavior of anions in electrochemical cells and their role in redox reactions.
- Computational Chemistry: Quantum mechanical calculations can be used to predict the properties and reactivity of anions.
Conclusion
A negatively charged ion is called an anion, and these negatively charged species are fundamental to chemistry, biology, and countless other scientific fields. Understanding their formation, properties, naming conventions, and wide-ranging applications is critical for students and professionals alike. From biological processes to materials science, anions' impact is pervasive and profound, making their study a fascinating and essential undertaking. This comprehensive exploration has provided a strong foundation for delving into the more intricate aspects of anion chemistry and their significant roles in our world. Continued exploration and research will undoubtedly reveal even more about the remarkable properties and diverse applications of these negatively charged particles.
Latest Posts
Latest Posts
-
Reverse List Python Without Inbuilt Function
Mar 18, 2025
-
A Current Of One Ampere Is Passed Through
Mar 18, 2025
-
Difference Between Earthing And Grounding And Neutral
Mar 18, 2025
-
What Is The Molar Mass Of Calcium Carbonate Caco3
Mar 18, 2025
-
How Many Lines Of Symmetry Does The Square Have
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about A Negatively Charged Ion Is Called A . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
