A Negatively Charged Ion Is Called
News Leon
Mar 15, 2025 · 6 min read
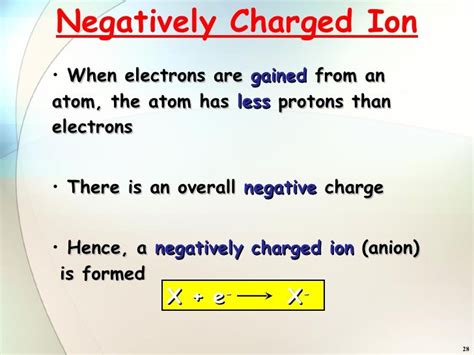
Table of Contents
A Negatively Charged Ion is Called an Anion: A Deep Dive into Ionic Chemistry
A negatively charged ion is called an anion. Understanding anions is fundamental to grasping many aspects of chemistry, physics, and even biology. This comprehensive guide will explore the world of anions, delving into their formation, properties, nomenclature, and their crucial roles in various fields. We'll also look at some common examples and practical applications.
What is an Ion?
Before we delve into the specifics of anions, let's establish a basic understanding of ions themselves. An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electrical charge. This charge imbalance is what distinguishes an ion from a neutral atom or molecule.
Cations vs. Anions
There are two main types of ions:
-
Cations: These are positively charged ions, formed when an atom loses one or more electrons. The loss of negatively charged electrons leaves the atom with a net positive charge. Common examples include sodium ions (Na⁺) and calcium ions (Ca²⁺).
-
Anions: As mentioned earlier, these are negatively charged ions, formed when an atom gains one or more electrons. The addition of negatively charged electrons results in a net negative charge. Examples include chloride ions (Cl⁻) and oxide ions (O²⁻).
Formation of Anions: The Electron Gain
The formation of an anion is a process driven by the atom's desire to achieve a stable electron configuration. Atoms are most stable when their outermost electron shell (valence shell) is full. This often means having eight electrons in the valence shell (the octet rule), although there are exceptions, particularly with elements in the first and second rows of the periodic table.
By gaining electrons, an atom can complete its valence shell and attain a lower energy state, making it more stable. This process often involves interactions with other atoms or molecules, particularly those that readily lose electrons (metals). The electronegativity of an atom plays a significant role. Highly electronegative atoms, such as those in Group 17 (halogens) and Group 16 (chalcogens), have a strong tendency to gain electrons and form anions.
Naming Anions: A Systematic Approach
The naming of anions follows a systematic approach based on the parent atom or polyatomic group:
-
Monatomic Anions: These are anions formed from a single atom. The name is formed by replacing the ending of the element's name with "-ide." For example:
- Chlorine (Cl) becomes chloride (Cl⁻)
- Oxygen (O) becomes oxide (O²⁻)
- Sulfur (S) becomes sulfide (S²⁻)
- Nitrogen (N) becomes nitride (N³⁻)
-
Polyatomic Anions: These are anions composed of two or more atoms covalently bonded together. Their naming conventions are more complex and often involve memorization or referencing a table. Some common polyatomic anions include:
- Sulfate (SO₄²⁻): Found in many salts and acids.
- Nitrate (NO₃⁻): A crucial component of fertilizers and explosives.
- Phosphate (PO₄³⁻): Essential for biological processes.
- Carbonate (CO₃²⁻): Found in limestone and other minerals.
- Hydroxide (OH⁻): A key component in many bases and reactions.
- Acetate (CH₃COO⁻): Common in organic chemistry and biochemistry.
Properties of Anions
The properties of anions vary greatly depending on the specific anion. However, some general characteristics are observable:
-
Negative Charge: The defining characteristic of all anions is their negative electrical charge. This charge influences their interactions with other ions and molecules.
-
Size: The size of an anion is generally larger than the corresponding neutral atom because of the added electrons. The increased electron-electron repulsion leads to an expansion of the electron cloud.
-
Reactivity: The reactivity of an anion depends on its electronic structure and the electronegativity of the constituent atoms. Some anions are highly reactive, readily participating in chemical reactions, while others are less reactive and more stable.
-
Solubility: The solubility of an anion in different solvents depends on its charge density and its interaction with the solvent molecules. Some anions are highly soluble in water, while others are insoluble.
Importance of Anions in Various Fields
Anions play crucial roles in a wide range of fields:
Biology and Medicine
-
Electrolyte Balance: Anions, such as chloride (Cl⁻) and bicarbonate (HCO₃⁻), are essential electrolytes, playing a critical role in maintaining fluid balance and nerve function in living organisms. Imbalances in anion concentrations can lead to serious health problems.
-
Enzyme Activity: Many enzymes require specific anions as cofactors for their activity. These anions can participate directly in the catalytic mechanism or help maintain the enzyme's structure.
-
Bone Structure: Phosphate (PO₄³⁻) ions are major components of bone mineral, providing structural strength and rigidity to the skeleton.
-
Cellular Processes: Anions are involved in numerous cellular processes, including signal transduction, transport across cell membranes, and energy production.
Chemistry and Industry
-
Salt Formation: Anions are essential components in the formation of salts. Salts are ionic compounds formed from the electrostatic attraction between cations and anions. These salts find widespread use in various applications.
-
Chemical Reactions: Anions participate in numerous chemical reactions, serving as reactants, catalysts, or products. Their reactivity is crucial in many industrial processes and chemical syntheses.
-
Material Science: Anions are used in the synthesis of various materials, such as ceramics, glasses, and polymers. Their properties significantly influence the final material's characteristics.
Environmental Science
-
Water Quality: The concentration of certain anions, such as nitrate (NO₃⁻) and sulfate (SO₄²⁻), in water bodies is an indicator of water quality and potential pollution. Excess amounts can have adverse environmental effects.
-
Soil Chemistry: Anions play a vital role in soil chemistry, influencing nutrient availability to plants and soil pH. Understanding anion behavior in soil is critical for sustainable agriculture.
Examples of Common Anions and Their Applications
Let's examine some specific examples of common anions and their applications:
-
Chloride (Cl⁻): Found in table salt (NaCl), it is essential for maintaining fluid balance in the body. It also has applications in the production of various chemicals and materials.
-
Sulfate (SO₄²⁻): Used in the production of fertilizers, it is also found in many minerals and plays a role in various industrial processes.
-
Nitrate (NO₃⁻): A crucial component of fertilizers, it is also a key ingredient in explosives. However, excessive nitrates in water can be harmful to human health and the environment.
-
Phosphate (PO₄³⁻): Essential for biological processes, it is also used in fertilizers, detergents, and food additives.
-
Carbonate (CO₃²⁻): Found in limestone and other minerals, it is used in various applications, including cement production and water treatment.
-
Hydroxide (OH⁻): A key component of bases, it is used in various industrial processes and chemical reactions.
Conclusion
Anions, the negatively charged ions, are fundamental building blocks of matter and play crucial roles in a wide variety of natural and industrial processes. Understanding their formation, properties, nomenclature, and diverse applications is vital for advancements in various scientific and technological fields. From the biological processes sustaining life to the industrial processes shaping our world, anions remain indispensable components of our understanding of the universe. Further exploration of this fascinating field will undoubtedly continue to reveal new insights and applications.
Latest Posts
Latest Posts
-
What Is The Electron Configuration For Ne
Mar 15, 2025
-
Tool Used To Detect Electric Charge
Mar 15, 2025
-
How Many Atoms Are In A Single Molecule Of H2o
Mar 15, 2025
-
Which Of The Following Is Not A Nucleophile
Mar 15, 2025
-
Define Paradox Of Value In Economics
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about A Negatively Charged Ion Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
