How Many Atoms Are In A Single Molecule Of H2o
News Leon
Mar 15, 2025 · 5 min read
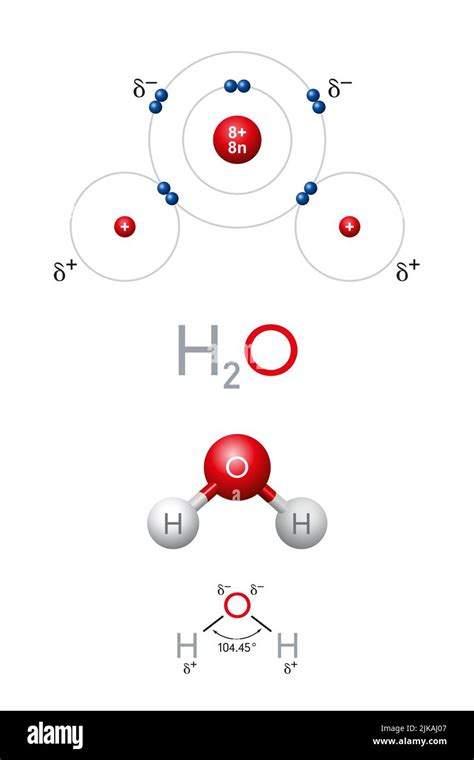
Table of Contents
How Many Atoms Are in a Single Molecule of H₂O? A Deep Dive into Water's Atomic Composition
Water. The elixir of life, the essential ingredient for all known life forms, and a deceptively simple molecule. But even something as seemingly basic as H₂O hides a universe of fascinating scientific details. One of the most fundamental questions we can ask about water is: how many atoms are in a single molecule of H₂O? The answer, while simple on the surface, opens the door to a deeper understanding of chemistry, molecular structure, and the very building blocks of our universe.
Deconstructing the Water Molecule: Atoms and Their Roles
The chemical formula for water, H₂O, is a concise representation of its atomic composition. Let's break it down:
-
H: This symbol represents hydrogen, one of the most abundant elements in the universe. Hydrogen atoms are incredibly small, possessing only one proton and one electron in their simplest form (protium). They are highly reactive and play a crucial role in many chemical processes.
-
₂: This subscript indicates that there are two hydrogen atoms present in each water molecule. These two hydrogen atoms are covalently bonded to the oxygen atom.
-
O: This symbol represents oxygen, another incredibly important element, vital for respiration and numerous other biological functions. An oxygen atom has eight protons and eight electrons in its neutral state. It's significantly larger than a hydrogen atom and more electronegative.
Therefore, a single molecule of H₂O contains three atoms: two hydrogen atoms and one oxygen atom.
Understanding Chemical Bonding: Covalent Bonds in H₂O
The three atoms in a water molecule aren't simply clustered together; they are connected by strong chemical bonds. In H₂O, these bonds are covalent bonds. This means that the atoms share electrons to achieve a more stable electronic configuration.
Specifically, each hydrogen atom shares one electron with the oxygen atom, forming a single covalent bond. The oxygen atom, in turn, shares two electrons, one with each hydrogen atom. This electron sharing creates a stable molecule where all atoms have a filled outer electron shell, satisfying the octet rule for oxygen and the duet rule for hydrogen.
This specific arrangement of atoms and bonds is what gives water its unique properties, such as its high surface tension, its ability to act as a universal solvent, and its high specific heat capacity.
Beyond the Basics: Isotopes and Variations
While the standard H₂O molecule consists of two protium atoms (¹H) and one oxygen-16 atom (¹⁶O), it's important to acknowledge that variations exist. This is due to the presence of isotopes.
-
Isotopes of Hydrogen: Hydrogen has two stable isotopes: deuterium (²H or D) and tritium (³H or T). Deuterium has one proton and one neutron, while tritium has one proton and two neutrons. Water molecules containing deuterium (D₂O, heavy water) and tritium (T₂O) have slightly different properties compared to regular water (H₂O).
-
Isotopes of Oxygen: Oxygen also has several stable isotopes, the most common being oxygen-16 (¹⁶O), oxygen-17 (¹⁷O), and oxygen-18 (¹⁸O). These isotopes differ in the number of neutrons in their nuclei. The presence of these oxygen isotopes affects the overall mass of the water molecule.
Therefore, while the fundamental answer remains three atoms per molecule, the precise mass and properties of a water molecule can vary slightly depending on the specific isotopes of hydrogen and oxygen present.
The Macro Perspective: Avogadro's Number and Moles
While we've focused on a single H₂O molecule, it's vital to understand how this translates to larger quantities of water. This is where Avogadro's number comes into play. Avogadro's number (approximately 6.022 x 10²³) represents the number of atoms or molecules in one mole of a substance.
One mole of water (18 grams) contains Avogadro's number of water molecules, each with three atoms. This means that 18 grams of water contain approximately 3 x (6.022 x 10²³) = 1.8066 x 10²⁴ atoms. This highlights the immense number of atoms even in a relatively small amount of water.
Applications and Significance: Why This Matters
Understanding the atomic composition of water, even at the most fundamental level, has profound implications across various scientific disciplines:
-
Chemistry: It provides the basis for understanding chemical reactions, bonding, and molecular structure. The simple water molecule serves as a foundational example for studying covalent bonding and intermolecular forces.
-
Biology: Water's unique properties, derived directly from its molecular structure, are crucial for life. Its role as a solvent, its high specific heat capacity, and its cohesive and adhesive properties are essential for biological processes.
-
Physics: The study of water's behavior at different temperatures and pressures helps us understand phase transitions, thermodynamics, and the properties of liquids. The study of heavy water (D₂O) also has applications in nuclear physics.
-
Environmental Science: Understanding the atomic and molecular structure of water is vital for studying water pollution, water purification, and the overall water cycle.
The Bigger Picture: Water's Role in the Universe
Water isn't just confined to Earth. Evidence suggests the presence of water ice on various celestial bodies within our solar system and beyond. Understanding the basic structure of the water molecule allows us to interpret spectral data and search for extraterrestrial life. The search for liquid water on other planets is often considered a key indicator of the potential for habitability.
The simplicity of the water molecule belies its profound complexity and significance. Its fundamental structure, composed of three atoms, is the foundation for understanding its unique properties and its crucial role in the universe.
Conclusion: Three Atoms, Infinite Possibilities
In conclusion, a single molecule of H₂O contains three atoms: two hydrogen atoms and one oxygen atom. This seemingly simple fact serves as the cornerstone for understanding the vast and intricate world of chemistry, biology, and the physical sciences. From the smallest scale of atomic interactions to the largest scale of planetary processes, the three atoms of water play an indispensable role. The ongoing exploration and study of water, from its molecular composition to its cosmological implications, promise to continue unveiling more profound insights into the workings of our universe. The simple question, "How many atoms are in a single molecule of H₂O?" ultimately leads to a journey of discovery, highlighting the beauty and power of scientific investigation.
Latest Posts
Latest Posts
-
Which Of The Following Is Polynomial
Mar 17, 2025
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Single Molecule Of H2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
