Is Boiling Water A Physical Change
News Leon
Mar 17, 2025 · 5 min read
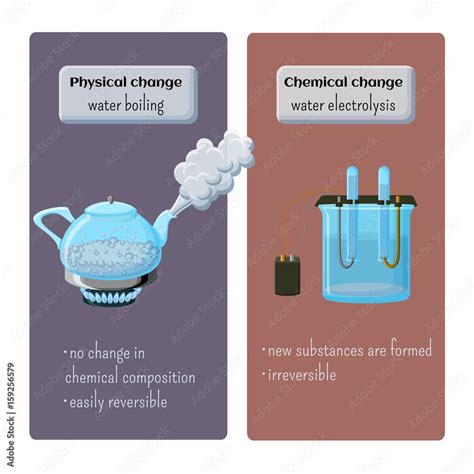
Table of Contents
Is Boiling Water a Physical Change? A Deep Dive into Phase Transitions
The question, "Is boiling water a physical change?" seems deceptively simple. At first glance, the answer is a resounding yes. However, a deeper exploration reveals a more nuanced understanding of physical and chemical changes, phase transitions, and the properties of water. This article will delve into the science behind boiling water, exploring the process from a molecular perspective and addressing common misconceptions. We'll examine why it's classified as a physical change, despite the dramatic transformation it undergoes.
Understanding Physical and Chemical Changes
Before we tackle the boiling water question, let's establish a clear understanding of the difference between physical and chemical changes.
Physical Changes: These are changes that affect the form or appearance of a substance but do not alter its chemical composition. The substance remains the same, just in a different state or form. Examples include:
- Changes of state: Melting, freezing, boiling, condensation, sublimation, and deposition.
- Shape changes: Cutting, bending, crushing.
- Dissolving: Salt dissolving in water.
Chemical Changes: These changes involve a rearrangement of atoms and molecules, resulting in the formation of new substances with different chemical properties. The original substance is transformed into something fundamentally different. Examples include:
- Burning: Combustion reactions.
- Rusting: Oxidation of iron.
- Cooking: Complex chemical reactions in food.
The Boiling Process: A Molecular Perspective
Boiling water is a phase transition, specifically a change from the liquid phase to the gaseous phase (vaporization). To understand why this is a physical change, let's examine what happens at the molecular level:
Water Molecules in the Liquid State
In liquid water, water molecules (H₂O) are constantly moving and colliding with each other. They are relatively close together, held by relatively weak intermolecular forces called hydrogen bonds. These bonds are constantly breaking and reforming, allowing the molecules to flow and take the shape of their container.
The Transition to the Gaseous State
When heat is applied, the kinetic energy of the water molecules increases. This increased energy overcomes the intermolecular forces holding the molecules together. At the boiling point (100°C or 212°F at standard atmospheric pressure), enough energy is present to break the majority of hydrogen bonds, and the molecules escape the liquid phase and enter the gaseous phase as water vapor (steam).
Crucially, the chemical composition remains unchanged. Each water molecule in the steam is still H₂O; it hasn't broken down into hydrogen and oxygen. The only change is the arrangement and distance between the molecules and their state of aggregation. This is the hallmark of a physical change.
Factors Affecting Boiling Point
The boiling point of water isn't a constant. Several factors influence it, including:
- Pressure: Lower atmospheric pressure lowers the boiling point. This is why water boils at a lower temperature at higher altitudes.
- Impurities: Dissolved substances can slightly elevate the boiling point. This is known as boiling point elevation.
- Heat transfer rate: The rate at which heat is applied affects how quickly the water reaches its boiling point, but not the boiling point itself.
Addressing Common Misconceptions
Despite the straightforward nature of the explanation, several misunderstandings frequently arise:
Misconception 1: Steam is different from water.
While steam and liquid water exist in different phases, they are chemically identical. Steam is simply water in its gaseous state.
Misconception 2: Boiling involves a chemical reaction.
No chemical reaction occurs during boiling. No new substances are formed. The process involves only a change in the physical state of water.
Misconception 3: The energy change is a chemical change indicator.
Energy changes accompany both physical and chemical changes. While energy is absorbed during boiling (endothermic process), this doesn't automatically classify it as a chemical change. Many physical changes also involve energy changes (e.g., melting ice).
Boiling Water and the Concept of Reversibility
Another key characteristic of physical changes is their reversibility. Boiling water can be reversed through condensation. As water vapor cools, the molecules lose kinetic energy, the intermolecular forces reassert themselves, and the water returns to its liquid state. This reversibility further reinforces the classification of boiling as a physical change.
The Importance of Precise Scientific Language
Using precise scientific language is crucial when discussing phase transitions and chemical reactions. Avoiding vague or misleading terms is essential for accurate communication and understanding. For example, saying "water transforms into steam" is more accurate than "water changes into steam," as the latter could imply a chemical transformation.
Applications and Real-World Examples
The physical change of boiling water has numerous applications in everyday life and industrial processes:
- Cooking: Boiling water is essential for cooking food, sterilizing utensils, and making beverages.
- Steam power generation: Boiling water drives turbines in power plants, converting thermal energy into mechanical energy.
- Sterilization: Boiling water effectively sterilizes instruments and surfaces by killing microorganisms.
- Distillation: Boiling is a crucial step in distillation, a separation technique used to purify liquids.
Conclusion
Boiling water is undeniably a physical change. While visually dramatic, the process involves only a change in the physical state of water, not its chemical composition. The water molecules remain H₂O throughout the transformation. Understanding this distinction is fundamental to grasping the concepts of physical and chemical changes and appreciating the intricate science behind everyday phenomena. By understanding the molecular interactions and the reversibility of the process, we can confidently classify boiling water as a prime example of a physical change. This knowledge is not only crucial for scientific understanding but also finds practical application in various fields, from cooking to industrial processes. The precise terminology used when describing such transformations is crucial for clear communication and precise scientific discourse.
Latest Posts
Latest Posts
-
What Is The Ph Of A Neutral Solution
Mar 17, 2025
-
Did The Ussr Imiss The Great Depression
Mar 17, 2025
-
Choose The Components Of A Respiratory Membrane
Mar 17, 2025
-
Dendrite Is To Axon As Is To
Mar 17, 2025
-
The Quotient Of A Number And 2
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
