What Is The Electron Configuration For Ne
News Leon
Mar 15, 2025 · 6 min read
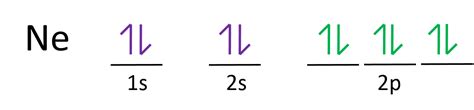
Table of Contents
What is the Electron Configuration for Neon? Understanding Noble Gas Stability
Neon (Ne), a vibrant element illuminating our signage and contributing to our understanding of atomic structure, holds a special place in chemistry. Its electron configuration is a cornerstone concept in understanding chemical behavior and the periodic table. This article delves deep into neon's electron configuration, exploring its significance, implications, and connections to other elements and principles of chemistry.
What is Electron Configuration?
Before we dive into neon's specific configuration, let's establish a fundamental understanding of electron configuration itself. The electron configuration of an atom describes the arrangement of electrons in its various energy levels and sublevels. This arrangement dictates the atom's chemical properties, reactivity, and its position on the periodic table.
Electrons occupy specific orbitals within energy levels. These orbitals are regions of space where there's a high probability of finding an electron. The principal energy levels (n=1, 2, 3, etc.) represent increasing energy, with electrons in higher levels possessing more energy. Within each principal energy level are sublevels (s, p, d, f), each capable of holding a specific number of electrons.
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The filling of these orbitals follows specific rules, including the Aufbau principle (filling orbitals in order of increasing energy), Hund's rule (maximizing unpaired electrons in a sublevel), and the Pauli exclusion principle (no two electrons can have the same four quantum numbers).
Neon's Electron Configuration: 1s²2s²2p⁶
Neon, with an atomic number of 10, possesses 10 electrons. Its electron configuration is written as 1s²2s²2p⁶. Let's break this down:
- 1s²: Two electrons occupy the first principal energy level (n=1) in the 's' sublevel. The 's' sublevel can hold a maximum of two electrons.
- 2s²: Two electrons occupy the second principal energy level (n=2) in the 's' sublevel.
- 2p⁶: Six electrons occupy the second principal energy level (n=2) in the 'p' sublevel. The 'p' sublevel can hold a maximum of six electrons.
This configuration represents a completely filled outer electron shell. This is crucial because it explains neon's chemical inertness.
The Significance of a Filled Outer Shell: Noble Gas Stability
Neon's completely filled outer electron shell (2s²2p⁶) is the key to understanding its chemical properties. Elements with completely filled outer electron shells are known as noble gases or inert gases. They are exceptionally stable and unreactive because they have no tendency to gain, lose, or share electrons to achieve a more stable configuration. The filled outer shell represents a state of maximum stability. It requires a significant amount of energy to disrupt this stable arrangement, thus explaining neon's reluctance to participate in chemical reactions.
This stability is a direct consequence of the electronic structure. A filled outer shell maximizes the electrostatic attraction between the positively charged nucleus and the negatively charged electrons. Any attempt to alter this arrangement would disrupt this optimal balance and require considerable energy input.
Comparing Neon's Electron Configuration to Other Elements
Comparing neon's electron configuration to other elements helps highlight the significance of its filled outer shell. Consider the elements preceding and following neon in the periodic table:
- Fluorine (F): Atomic number 9; electron configuration 1s²2s²2p⁵. Fluorine has one electron short of a complete outer shell and thus is highly reactive, readily gaining an electron to achieve a stable octet (eight electrons in the outer shell).
- Sodium (Na): Atomic number 11; electron configuration 1s²2s²2p⁶3s¹. Sodium has one electron more than a complete outer shell. It readily loses this electron to achieve the stable configuration of neon, resulting in a +1 ion.
This comparison clearly demonstrates that the filled outer shell of neon contributes to its exceptional stability and chemical inertness, unlike its neighboring elements, which are highly reactive.
Neon's Applications: Leveraging its Inertness
Neon's chemical inertness is the basis for many of its practical applications:
- Neon signs: Neon's unique ability to emit a bright reddish-orange glow when excited by an electrical current makes it ideal for signage. The color can be varied by using different gases within the glass tubes.
- Lasers: Neon's spectral properties enable its use in neon lasers, which produce coherent light used in various applications, including barcode scanning and scientific research.
- Cryogenics: Liquid neon, obtained by liquefying gaseous neon at very low temperatures, is used as a refrigerant in certain applications due to its low boiling point.
- High-Voltage Indicators: Neon's ability to conduct electricity at high voltages makes it useful in electrical indicator lights.
These applications highlight how the unique properties derived from its electron configuration translate into practical uses.
Advanced Concepts and Extensions
While the basic electron configuration explains much of neon's behavior, more advanced concepts provide a deeper understanding:
- Quantum Numbers: A complete description of an electron requires four quantum numbers: principal (n), azimuthal (l), magnetic (ml), and spin (ms). These numbers define the electron's energy, orbital shape, orbital orientation, and spin.
- Orbital Diagrams: These diagrams illustrate the arrangement of electrons within individual orbitals, including their spin. For neon, this would show each orbital within the 1s, 2s, and 2p sublevels fully occupied.
- Electron-Electron Repulsion: While the simplified model assumes electrons are independent, in reality, they repel each other. This repulsion slightly influences the energy levels and electron distributions.
- Spectroscopy: Analyzing the light emitted or absorbed by neon atoms provides further insight into the electronic transitions and energy levels within the atom. The sharp spectral lines of neon are characteristic of its electron configuration.
Conclusion: Neon's Electron Configuration and its Broader Significance
Neon's electron configuration, 1s²2s²2p⁶, is more than just a notation; it's a key to understanding its unique properties and behavior. The completely filled outer shell leads to exceptional stability and inertness, underpinning its applications and highlighting the fundamental principles of atomic structure and chemical reactivity. By studying neon's configuration, we gain a deeper appreciation for the connection between electron arrangement, chemical properties, and the broader principles that govern the behavior of matter. The principles learned from studying neon can then be applied to understanding the behavior of other elements, furthering our understanding of the periodic table and chemical bonding. Further exploration into advanced concepts relating to quantum numbers, orbital diagrams, and spectroscopy allows for a more nuanced and comprehensive grasp of atomic structure and its implications.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Ne . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
