2 5 Dimethyl 4 2 Methylpropyl Octane
News Leon
Mar 31, 2025 · 5 min read
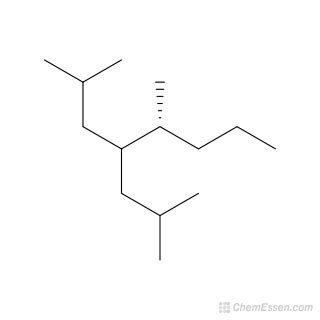
Table of Contents
2,5-Dimethyl-4-(2-methylpropyl)octane: A Deep Dive into its Structure, Properties, and Potential Applications
2,5-Dimethyl-4-(2-methylpropyl)octane, also known as 2,5-dimethyl-4-isobutyl octane, is a branched alkane hydrocarbon with the chemical formula C<sub>13</sub>H<sub>28</sub>. While not as widely studied or utilized as some other hydrocarbons, understanding its structure, properties, and potential applications provides valuable insight into the broader world of organic chemistry and its industrial implications. This comprehensive article will delve into these aspects, exploring its unique characteristics and potential future roles.
Understanding the Structure of 2,5-Dimethyl-4-(2-methylpropyl)octane
The name itself reveals much about the molecule's structure. Let's break it down:
- Octane: This indicates a parent chain of eight carbon atoms.
- 2,5-Dimethyl: This signifies two methyl groups (CH<sub>3</sub>) attached to the second and fifth carbon atoms of the octane chain.
- 4-(2-methylpropyl): This describes a 2-methylpropyl group (also known as an isobutyl group) attached to the fourth carbon atom of the octane chain. The 2-methylpropyl group itself consists of a propane chain with a methyl group on the second carbon.
This intricate arrangement results in a highly branched structure. The branching significantly impacts its physical and chemical properties, differentiating it from its linear isomers. Visualizing this structure using molecular modeling software or drawing it out using standard organic chemistry notation is crucial for a complete understanding. The branched nature leads to steric hindrance, which plays a vital role in its reactivity and interactions with other molecules.
Isomerism and its Significance
It's important to note that numerous isomers exist for C<sub>13</sub>H<sub>28</sub>. Isomers are molecules with the same molecular formula but different structural arrangements. The specific arrangement of atoms in 2,5-dimethyl-4-(2-methylpropyl)octane dictates its unique properties. Different isomers will exhibit varying boiling points, melting points, densities, and reactivity. The study of isomerism is fundamental to organic chemistry and understanding the behavior of specific molecules like this one.
The existence of many isomers highlights the complexity of organic chemistry and the importance of precise nomenclature to avoid ambiguity. Understanding the various types of isomerism (structural, geometric, etc.) helps in predicting the properties and potential applications of different molecules.
Physical and Chemical Properties
The branched structure of 2,5-dimethyl-4-(2-methylpropyl)octane profoundly influences its physical and chemical properties.
Physical Properties
-
Boiling Point: Due to its branched structure, it likely possesses a lower boiling point compared to its linear isomers. Branched alkanes have weaker intermolecular forces (London dispersion forces) than their linear counterparts due to reduced surface area contact. A lower boiling point indicates that less energy is needed to overcome these forces and transition from liquid to gas.
-
Melting Point: Similar to the boiling point, the melting point will also be affected by the branching. The precise melting point would need experimental determination, but it's expected to be lower than that of straight-chain alkanes with the same number of carbon atoms.
-
Density: The density is likely slightly lower than that of linear alkanes due to the less efficient packing of the branched molecules.
-
Solubility: Like most alkanes, 2,5-dimethyl-4-(2-methylpropyl)octane is essentially insoluble in water due to its nonpolar nature. It's, however, soluble in many organic solvents.
Chemical Properties
-
Combustion: Like all alkanes, it readily undergoes combustion in the presence of oxygen, producing carbon dioxide, water, and heat. This property makes it a potential fuel source, though its specific energy density would need to be calculated and compared to other fuels.
-
Halogenation: It can undergo halogenation reactions with halogens (like chlorine or bromine) under appropriate conditions, resulting in the substitution of hydrogen atoms with halogen atoms. The reaction rate and regioselectivity (preference for substitution at specific positions) would depend on the reaction conditions and the specific halogen used.
-
Oxidation: Under strong oxidizing conditions, it can be oxidized to produce various oxygenated organic compounds. The products would depend on the oxidizing agent and reaction conditions.
-
Reactivity: Generally, alkanes are relatively unreactive due to the strong C-C and C-H bonds. However, under specific conditions and with appropriate catalysts, reactions such as isomerization, cracking, and reforming are possible.
Potential Applications
While 2,5-dimethyl-4-(2-methylpropyl)octane might not have widespread individual applications like some other hydrocarbons, its properties make it relevant in several contexts:
Fuel Component
Its combustion properties suggest potential use as a component in fuel blends. The branched structure may affect the fuel's performance characteristics such as octane rating, volatility, and combustion efficiency. Further research would be needed to optimize its use in fuel formulations.
Solvent
Its solubility in various organic solvents could make it a component in specialized solvent mixtures for specific industrial processes. However, its application as a solvent would likely be niche due to the availability of more common and readily available solvents.
Research Applications
This compound could serve as a valuable tool in chemical research, particularly in studies involving branched alkanes, their properties, and their reactivity. It can help scientists understand the impact of branching on molecular behavior and reactivity. This understanding is crucial for designing and synthesizing new molecules with specific properties.
Lubricant Component
The branched structure could influence its viscosity and lubricating properties. It could potentially be a component in specialized lubricating oils, though more extensive research would be required to assess its performance compared to existing lubricants.
Conclusion: Further Research and Exploration
2,5-Dimethyl-4-(2-methylpropyl)octane, while not a widely used chemical, presents interesting possibilities for future research and potential applications. Further investigation into its precise physical and chemical properties, including detailed thermodynamic data, is essential for a comprehensive understanding of its potential uses. Comparative studies with its isomers and other similar hydrocarbons are crucial for determining its advantages and disadvantages in different contexts.
This molecule represents a valuable example of the vast number of organic compounds with unique properties, highlighting the ongoing need for research and development in organic chemistry and its diverse applications. The branched nature of this alkane showcases the complexities of isomerism and the profound impact of molecular structure on physical and chemical properties, making it a worthy subject of continuing study within the field of hydrocarbon chemistry. Its potential applications in fuel blends, solvent mixtures, and specialized lubricants require further investigation to determine its viability and optimize its performance. The data gathered through such studies will contribute to the overall advancement of chemical science and technology.
Latest Posts
Latest Posts
-
All Of The Following Refer To Mitosis Except
Apr 01, 2025
-
Which Of The Following Temperatures Is The Coldest
Apr 01, 2025
-
A Short Term Unsecured Promissory Note Issued By A Company Is
Apr 01, 2025
-
Adjacent Angles Whose Sum In 180 Degrees
Apr 01, 2025
-
Lewis Dot Structure For Magnesium Chloride
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about 2 5 Dimethyl 4 2 Methylpropyl Octane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
