Write The Iupac Name Of The Following Compounds
News Leon
Mar 19, 2025 · 7 min read
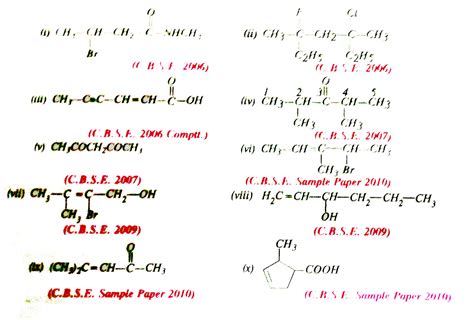
Table of Contents
Mastering IUPAC Nomenclature: A Comprehensive Guide with Examples
The International Union of Pure and Applied Chemistry (IUPAC) nomenclature is the globally accepted standard for naming chemical compounds. This system ensures clarity and avoids ambiguity, allowing chemists worldwide to communicate effectively about chemical structures. Mastering IUPAC nomenclature is crucial for anyone studying or working in the field of chemistry. This comprehensive guide will delve into the rules and principles of IUPAC nomenclature, providing numerous examples to solidify your understanding. We will cover various functional groups, complexities in chain structures, and isomerism, equipping you with the skills to name a wide array of organic and inorganic compounds.
Understanding the Fundamentals
Before diving into specific examples, let's establish the foundational principles governing IUPAC nomenclature:
- Parent Chain: Identify the longest continuous carbon chain in the molecule. This chain forms the basis of the compound's name.
- Substituents: Any atoms or groups of atoms attached to the parent chain are considered substituents.
- Numbering: Number the carbon atoms in the parent chain, starting from the end that gives the substituents the lowest possible numbers.
- Prefixes: Use prefixes (e.g., di-, tri-, tetra-) to indicate the number of times a substituent appears.
- Suffixes: The suffix indicates the principal functional group present in the molecule.
Alkanes: The Foundation
Alkanes are saturated hydrocarbons with the general formula C<sub>n</sub>H<sub>2n+2</sub>. They form the foundation for naming many other organic compounds. Here’s how to name simple alkanes:
- Methane (CH<sub>4</sub>): One carbon atom.
- Ethane (C<sub>2</sub>H<sub>6</sub>): Two carbon atoms.
- Propane (C<sub>3</sub>H<sub>8</sub>): Three carbon atoms.
- Butane (C<sub>4</sub>H<sub>10</sub>): Four carbon atoms.
- Pentane (C<sub>5</sub>H<sub>12</sub>): Five carbon atoms.
- Hexane (C<sub>6</sub>H<sub>14</sub>): Six carbon atoms.
- Heptane (C<sub>7</sub>H<sub>16</sub>): Seven carbon atoms.
- Octane (C<sub>8</sub>H<sub>18</sub>): Eight carbon atoms.
- Nonane (C<sub>9</sub>H<sub>20</sub>): Nine carbon atoms.
- Decane (C<sub>10</sub>H<sub>22</sub>): Ten carbon atoms.
Branched Alkanes: Incorporating Substituents
When dealing with branched alkanes, the process becomes slightly more complex:
- Identify the longest continuous carbon chain: This forms the parent alkane.
- Identify and name the substituents: These are the branches attached to the parent chain. Common substituents include methyl (CH<sub>3</sub>-), ethyl (CH<sub>3</sub>CH<sub>2</sub>-), propyl (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>-), and butyl (CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>CH<sub>2</sub>-). Isopropyl and tert-butyl are also common branched substituents.
- Number the carbon atoms in the parent chain: Start numbering from the end that gives the substituents the lowest possible numbers. If there are multiple substituents, prioritize the lowest number for the first substituent encountered.
- List the substituents alphabetically: Use prefixes (di-, tri-, tetra-) to indicate multiple occurrences of the same substituent. However, remember that alphabetization precedes the prefix (e.g., ethyl before dimethyl).
- Combine the information: Write the name as: (Substituent names in alphabetical order) + (Parent alkane name).
Example 1:
Consider the branched alkane with the structure: CH<sub>3</sub>CH(CH<sub>3</sub>)CH<sub>2</sub>CH<sub>3</sub>
- Longest chain: Four carbons (butane).
- Substituent: One methyl group (CH<sub>3</sub>-) on the second carbon.
- Name: 2-Methylbutane
Example 2:
Consider the branched alkane with the structure: CH<sub>3</sub>CH(CH<sub>3</sub>)CH(CH<sub>3</sub>)CH<sub>3</sub>
- Longest chain: Four carbons (butane).
- Substituents: Two methyl groups on carbons 2 and 3.
- Name: 2,3-Dimethylbutane
Example 3 (Illustrating Alphabetical Ordering and Prefix Usage):
Consider the compound: CH<sub>3</sub>CH<sub>2</sub>CH(CH<sub>2</sub>CH<sub>3</sub>)CH(CH<sub>3</sub>)<sub>2</sub>
- Longest chain: Five carbons (pentane).
- Substituents: One ethyl group on carbon 3 and two methyl groups on carbon 4. Alphabetize ethyl before methyl.
- Name: 3-Ethyl-4,4-dimethylpentane
Alkenes and Alkynes: Incorporating Unsaturation
Alkenes contain carbon-carbon double bonds (C=C), while alkynes contain carbon-carbon triple bonds (C≡C). Their IUPAC names are derived similarly to alkanes, but with important additions:
- Identify the longest continuous chain containing the multiple bond(s).
- Number the carbon atoms to give the multiple bond(s) the lowest possible number. The numbering begins from the end closest to the double or triple bond.
- Use the suffix "-ene" for alkenes and "-yne" for alkynes.
- Indicate the position of the multiple bond(s) using the number of the first carbon involved in the bond.
Example 4 (Alkene):
CH<sub>2</sub>=CHCH<sub>2</sub>CH<sub>3</sub>
- Longest chain: Four carbons (butane).
- Double bond: Between carbons 1 and 2.
- Name: 1-Butene
Example 5 (Alkyne):
CH≡CCH<sub>2</sub>CH<sub>3</sub>
- Longest chain: Four carbons (butane).
- Triple bond: Between carbons 1 and 2.
- Name: 1-Butyne
Compounds with Multiple Functional Groups:
When a molecule contains multiple functional groups, a priority system is used to determine the principal functional group, which dictates the suffix. The other functional groups are treated as substituents and named as prefixes. The priority order generally follows the order: carboxylic acids > anhydrides > esters > amides > nitriles > aldehydes > ketones > alcohols > amines > alkenes > alkynes > alkyl halides > alkanes.
Example 6 (Alcohol and Alkyl Halide):
Consider the compound: CH<sub>3</sub>CH(OH)CH<sub>2</sub>Br
- Principal functional group: Alcohol (-OH)
- Substituent: Bromo-
- Numbering: Start numbering from the carbon atom bearing the –OH group.
- Name: 3-Bromopropan-1-ol
Cyclic Compounds:
Cyclic compounds, including cycloalkanes, cycloalkenes, and cycloalkynes, are named using the prefix "cyclo-" before the alkane name, indicating the ring structure. Substituents on the ring are numbered to give the lowest possible numbers.
Example 7 (Cycloalkane):
Consider a cyclohexane ring with a methyl group on carbon 1 and an ethyl group on carbon 3.
- Parent structure: Cyclohexane
- Substituents: Methyl and ethyl
- Name: 1-Methyl-3-ethylcyclohexane
Aromatic Compounds:
Aromatic compounds, particularly benzene derivatives, have their own naming conventions. Many are named using common names (e.g., toluene, phenol, aniline), but IUPAC nomenclature can also be applied. When using IUPAC nomenclature for benzene derivatives, the numbering of the ring starts from the substituent that is listed first alphabetically.
Example 8 (Benzene Derivative):
Consider a benzene ring with a methyl group and a chloro group.
- Parent structure: Benzene
- Substituents: Methyl and chloro (chlorine takes priority alphabetically).
- Name: 1-Chloro-2-methylbenzene (or ortho-chlorotoluene using common naming)
Stereoisomers:
Stereoisomers possess the same molecular formula and connectivity but differ in the spatial arrangement of atoms. IUPAC nomenclature includes systems for specifying the configuration of stereoisomers, such as E/Z nomenclature for alkenes and R/S nomenclature for chiral centers. These are advanced aspects of nomenclature and require a more in-depth understanding of stereochemistry.
In Conclusion:
This comprehensive guide provides a strong foundation in IUPAC nomenclature, enabling you to name a broad spectrum of organic compounds. Remember that practice is key to mastering this system. Work through numerous examples, and gradually increase the complexity of the molecules you attempt to name. While this guide has covered the basics extensively, further exploration of specific functional groups and advanced stereochemical concepts will deepen your understanding and ability to apply IUPAC nomenclature effectively. Consistent application and practice will make you proficient in this vital aspect of chemistry.
Latest Posts
Latest Posts
-
What Event Had An Enormous Effect On Us Workplace Safety
Mar 19, 2025
-
What Is The Formula For Magnesium Acetate
Mar 19, 2025
-
Converse Of Alternate Exterior Angles Theorem
Mar 19, 2025
-
The Distance Between Adjacent Crests Is Called
Mar 19, 2025
-
Why Are Phenols More Acidic Than Alcohols
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Write The Iupac Name Of The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
