Why Are Phenols More Acidic Than Alcohols
News Leon
Mar 19, 2025 · 5 min read
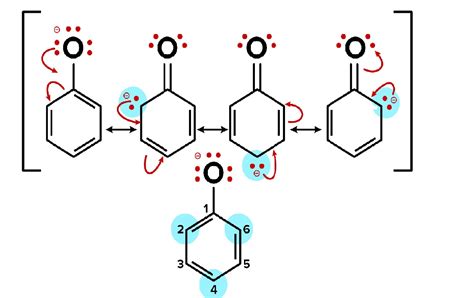
Table of Contents
Why Are Phenols More Acidic Than Alcohols? A Deep Dive into Acidity and Stability
Phenols and alcohols, both featuring a hydroxyl (-OH) group, might seem similar at first glance. However, a crucial difference lies in their acidity: phenols are significantly more acidic than alcohols. This seemingly simple observation opens a door to a deeper understanding of organic chemistry, encompassing resonance, inductive effects, and the stability of conjugate bases. This comprehensive article will dissect the reasons behind this acidity difference, exploring the underlying chemical principles in detail.
Understanding Acidity: The Role of Conjugate Bases
The acidity of a compound is determined by its willingness to donate a proton (H⁺). A stronger acid readily donates its proton, resulting in a more stable conjugate base. Conversely, a weaker acid holds onto its proton more tightly, forming a less stable conjugate base. The key to understanding why phenols are more acidic than alcohols lies in analyzing the stability of their respective conjugate bases – phenoxide and alkoxide ions.
The Phenoxide Ion: Resonance Stabilization
When a phenol loses a proton, it forms a phenoxide ion. This ion possesses a negative charge localized on the oxygen atom. Crucially, this negative charge is not confined to the oxygen; it's delocalized across the benzene ring through resonance. The benzene ring's conjugated π-system allows for the negative charge to be distributed among the carbon atoms in the ring. This delocalization significantly stabilizes the phenoxide ion.
Resonance Structures: Multiple resonance structures can be drawn for the phenoxide ion, demonstrating the distribution of the negative charge. This resonance stabilization is a significant factor in enhancing the phenol's acidity. The negative charge is not concentrated on a single atom, thereby reducing electron-electron repulsion and lowering the overall energy of the ion.
The Alkoxide Ion: Lack of Resonance Stabilization
Alcohols, upon losing a proton, form alkoxide ions. Unlike the phenoxide ion, the alkoxide ion lacks the extended π-system necessary for resonance stabilization. The negative charge remains localized on the oxygen atom. This localized charge leads to higher electron-electron repulsion, making the alkoxide ion considerably less stable than the phenoxide ion.
Comparison of Conjugate Bases: The contrasting stability between phenoxide and alkoxide ions directly explains the acidity difference. The greater stability of the phenoxide ion makes it easier for phenol to donate a proton, thus making it a stronger acid.
Inductive Effects: A Secondary Contributor
While resonance stabilization is the primary reason for the increased acidity of phenols, inductive effects also play a secondary role. The benzene ring's electron-withdrawing inductive effect pulls electron density away from the oxygen atom in the hydroxyl group. This effect makes the O-H bond slightly weaker, facilitating proton donation.
Electron-Withdrawing Groups: Enhancing Acidity
The presence of electron-withdrawing groups (EWGs) on the benzene ring further enhances the acidity of phenols. These groups, such as nitro (-NO₂) or halogens (-Cl, -Br, -I), intensify the inductive effect, further stabilizing the phenoxide ion and increasing the acidity. The stronger the electron-withdrawing group, the greater the increase in acidity.
Electron-Donating Groups: Reducing Acidity
Conversely, electron-donating groups (EDGs), such as methyl (-CH₃) or methoxy (-OCH₃), decrease the acidity of phenols. These groups push electron density towards the oxygen atom, making the O-H bond stronger and hindering proton donation.
The pKa Values: Quantitative Evidence
The difference in acidity between phenols and alcohols is quantitatively expressed through their pKa values. The pKa is a measure of acidity, with lower pKa values indicating stronger acids. Typically, phenols have pKa values ranging from 9 to 10, while alcohols have pKa values around 16. This significant difference (approximately 6-7 pKa units) underscores the substantially greater acidity of phenols.
Factors Affecting Phenol Acidity: A Deeper Look
Several factors can influence the acidity of phenols beyond the fundamental resonance and inductive effects:
Steric Hindrance: Impact on Conjugate Base Stability
While not as prominent as resonance, steric hindrance can influence the stability of the phenoxide ion. Bulky substituents near the hydroxyl group can hinder the delocalization of the negative charge, slightly decreasing the acidity. However, this effect is generally less significant than the resonance stabilization.
Solvent Effects: The Role of the Environment
The solvent in which the phenol is dissolved also plays a role in its acidity. Protic solvents, which can form hydrogen bonds, can stabilize both the phenol and the phenoxide ion, but the effect on the phenoxide ion is generally greater. This can affect the measured pKa value.
Practical Applications: The Significance of Phenol Acidity
The increased acidity of phenols compared to alcohols has significant implications in various applications:
Pharmaceuticals: Drug Design and Development
Many pharmaceuticals contain phenol groups. The acidity of phenols allows for various chemical modifications and interactions, crucial in drug design and development.
Polymers and Materials Science: Polymer Synthesis and Modification
Phenols are frequently used in the synthesis of polymers and other materials. Their acidity can be exploited in various reactions, leading to desirable material properties.
Environmental Science: Pollution Control and Remediation
Phenols' reactivity due to their acidity can be harnessed in various environmental applications, including pollution control and remediation.
Conclusion: A Recap of Phenol's Enhanced Acidity
In conclusion, the enhanced acidity of phenols compared to alcohols is primarily attributed to the significant resonance stabilization of the phenoxide ion. The delocalization of the negative charge across the benzene ring drastically reduces electron-electron repulsion and lowers the energy of the conjugate base, making phenol a stronger acid. While inductive effects and other factors contribute, resonance stabilization remains the dominant influence determining the acidity difference. Understanding this fundamental difference has broad implications across various scientific fields, highlighting the importance of this seemingly simple observation in organic chemistry.
Latest Posts
Latest Posts
-
Barium Chloride And Sodium Sulphate Reaction
Mar 19, 2025
-
Is A Webcam Input Or Output
Mar 19, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 19, 2025
-
Which Of The Earths Layers Is The Thinnest
Mar 19, 2025
-
Three Particles Are Fixed On An X Axis
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Why Are Phenols More Acidic Than Alcohols . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
