What Type Of Bonding Involves The Unequal Sharing Of Electrons
News Leon
Mar 19, 2025 · 6 min read
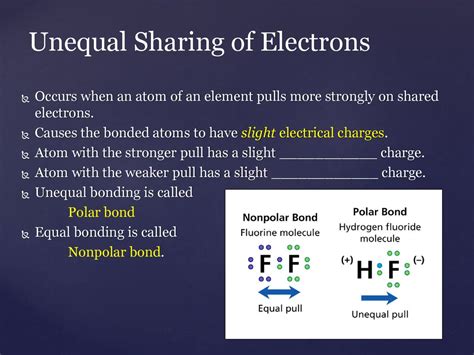
Table of Contents
What Type of Bonding Involves the Unequal Sharing of Electrons?
Polar covalent bonding is the type of bonding that involves the unequal sharing of electrons between two atoms. This unequal sharing arises from a difference in electronegativity between the atoms involved. Understanding polar covalent bonds is crucial to comprehending the properties of a vast array of molecules and materials, from simple water molecules to complex biological macromolecules. This article delves deep into the concept, exploring its characteristics, consequences, and applications.
Understanding Electronegativity: The Root of Polarity
Before diving into polar covalent bonds, it's essential to grasp the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Atoms with high electronegativity strongly attract electrons, while those with low electronegativity have a weaker pull. The electronegativity difference between two atoms dictates the nature of the bond they form.
The Electronegativity Scale
The most commonly used electronegativity scale is the Pauling scale, developed by Linus Pauling. This scale assigns values to elements, with fluorine (F), the most electronegative element, having a value of 4.0. Other elements have values ranging from less than 1 to slightly over 3, reflecting their varying abilities to attract electrons.
Electronegativity and Bond Type
The difference in electronegativity (ΔEN) between two atoms determines the type of bond they form:
-
ΔEN = 0: Nonpolar covalent bond – electrons are shared equally between atoms of similar electronegativity. Examples include bonds within diatomic molecules like O₂ and N₂.
-
0 < ΔEN < 1.7: Polar covalent bond – electrons are shared unequally, leading to a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. This creates a dipole moment. Examples include bonds in water (H₂O) and ammonia (NH₃).
-
ΔEN ≥ 1.7: Ionic bond – electrons are essentially transferred from the less electronegative atom to the more electronegative atom, resulting in the formation of ions (cations and anions) held together by electrostatic attraction. Examples include NaCl (sodium chloride) and MgO (magnesium oxide).
The Characteristics of Polar Covalent Bonds
Polar covalent bonds exhibit several key characteristics:
-
Unequal Electron Distribution: The most defining feature is the unequal sharing of electrons. The bonding electrons spend more time closer to the more electronegative atom.
-
Dipole Moment: The unequal electron distribution creates a dipole moment, a vector quantity representing the separation of positive and negative charges within the molecule. The direction of the dipole moment points from the partially positive atom (δ+) to the partially negative atom (δ-). This dipole moment is often represented by an arrow with a plus sign at the tail.
-
Polar Molecules: Molecules containing polar covalent bonds are often polar molecules themselves, although the overall polarity depends on the molecular geometry. If the individual bond dipoles don't cancel each other out, the molecule has a net dipole moment.
-
Higher Boiling and Melting Points: Compared to nonpolar molecules of similar size, polar molecules typically have higher boiling and melting points due to the stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding) arising from their polarity.
-
Solubility: Polar molecules tend to dissolve well in polar solvents (like water) due to the attraction between the dipoles. They are generally less soluble in nonpolar solvents.
Examples of Polar Covalent Bonds
Numerous examples of polar covalent bonds exist in nature and synthetic materials. Some prominent instances include:
-
Water (H₂O): The oxygen atom is significantly more electronegative than the hydrogen atoms, resulting in polar O-H bonds. The bent molecular geometry of water ensures that the bond dipoles don't cancel out, leading to a polar molecule.
-
Ammonia (NH₃): Similar to water, the nitrogen atom is more electronegative than the hydrogen atoms, creating polar N-H bonds. The pyramidal shape of ammonia results in a net dipole moment.
-
Hydrogen Fluoride (HF): The exceptionally high electronegativity of fluorine makes the H-F bond highly polar.
-
Carbonyl Group (C=O): The oxygen atom's higher electronegativity than carbon creates a polar C=O bond, a crucial functional group in many organic molecules such as aldehydes, ketones, and carboxylic acids.
-
Hydroxyl Group (-OH): This group, found in alcohols and other organic compounds, features a polar O-H bond.
Consequences of Polar Covalent Bonding
The unequal sharing of electrons in polar covalent bonds has significant consequences for the properties and behavior of molecules:
-
Hydrogen Bonding: A particularly strong type of dipole-dipole interaction occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) interacts with another electronegative atom in a nearby molecule. This hydrogen bonding is responsible for many of water's unique properties, including its high boiling point and its ability to act as a universal solvent.
-
Solubility and Miscibility: Polarity significantly influences a molecule's solubility. Polar molecules tend to dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents. This principle is encapsulated in the adage "like dissolves like."
-
Reactivity: Polar molecules are often more reactive than nonpolar molecules because the partial charges can participate in various chemical interactions, such as acid-base reactions and nucleophilic attacks.
-
Physical Properties: The presence of polar bonds influences physical properties like melting point, boiling point, density, and viscosity.
Distinguishing Polar Covalent from Other Bond Types
It's crucial to differentiate polar covalent bonds from other types of chemical bonds:
-
Nonpolar Covalent Bonds: These bonds involve the equal sharing of electrons between atoms with similar electronegativities. They lack a dipole moment.
-
Ionic Bonds: In ionic bonds, electrons are transferred from one atom to another, forming ions with full charges. The resulting attraction between oppositely charged ions is much stronger than the dipole-dipole interaction in polar covalent bonds.
-
Metallic Bonds: Metallic bonds involve a "sea" of delocalized electrons shared among a lattice of metal atoms. This type of bonding is unique to metals and alloys.
Applications of Polar Covalent Bonding
Understanding polar covalent bonds is essential in various fields:
-
Chemistry: Predicting the properties and reactivity of molecules.
-
Biology: Understanding the structure and function of biological molecules like proteins, DNA, and carbohydrates, which rely heavily on polar covalent bonds. The polarity of water is essential for life processes.
-
Materials Science: Designing materials with specific properties, such as solubility, conductivity, and reactivity, by manipulating the types and arrangements of polar covalent bonds.
-
Medicine: Developing drugs and therapeutic agents that interact with specific biological targets through polar interactions.
Conclusion
Polar covalent bonding, driven by the unequal sharing of electrons due to differences in electronegativity, is a fundamental concept in chemistry. Its understanding is crucial for explaining the properties and behaviors of a wide array of molecules and materials. From the unique properties of water to the intricate functions of biological macromolecules and the design of advanced materials, the impact of polar covalent bonding is vast and profound. The ability to predict and manipulate the polarity of bonds is vital in numerous scientific and technological applications. Further exploration into this area continues to unlock new possibilities in various fields.
Latest Posts
Latest Posts
-
Which Of The Following Is Not A Type Of Rna
Mar 20, 2025
-
An External User Of Accounting Information
Mar 20, 2025
-
Computer Programs Are Also Known As
Mar 20, 2025
-
Atp Is Called The Energy Currency Of The Cell Because
Mar 20, 2025
-
Which Of The Following Is An Even Function
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bonding Involves The Unequal Sharing Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
