Barium Chloride And Sodium Sulphate Reaction
News Leon
Mar 19, 2025 · 5 min read
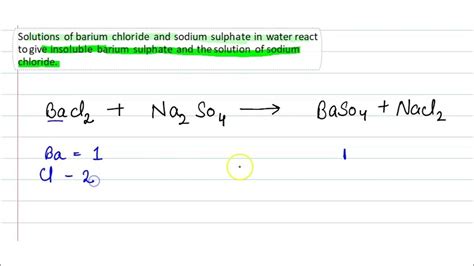
Table of Contents
Barium Chloride and Sodium Sulphate: A Deep Dive into a Classic Double Displacement Reaction
The reaction between barium chloride (BaCl₂) and sodium sulphate (Na₂SO₄) is a quintessential example of a double displacement reaction, also known as a metathesis reaction. This seemingly simple reaction offers a wealth of learning opportunities, spanning various aspects of chemistry, from stoichiometry and solubility rules to practical applications and safety considerations. This comprehensive article delves into the intricacies of this reaction, exploring its mechanism, observations, applications, and safety precautions.
Understanding the Reaction: A Molecular Perspective
At its core, the reaction between barium chloride and sodium sulphate involves the exchange of ions between two soluble ionic compounds. When aqueous solutions of barium chloride and sodium sulphate are mixed, the barium (Ba²⁺) ions and sulphate (SO₄²⁻) ions interact, forming an insoluble precipitate of barium sulphate (BaSO₄). Simultaneously, the sodium (Na⁺) and chloride (Cl⁻) ions remain dissolved in the solution as spectator ions, not participating directly in the net ionic reaction.
The balanced chemical equation for this reaction is:
BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq)
This equation clearly shows the exchange of ions: barium ions from barium chloride combine with sulphate ions from sodium sulphate to form barium sulphate, while sodium ions and chloride ions remain in solution.
The Role of Solubility Rules
The formation of the barium sulphate precipitate is governed by solubility rules. Barium sulphate is notoriously insoluble in water, a key factor driving this reaction to completion. This insolubility is due to the strong lattice energy of the barium sulphate crystal, making it energetically favorable for barium and sulphate ions to form a solid rather than remain dissolved in solution. Understanding solubility rules is crucial in predicting the outcome of many double displacement reactions.
Observing the Reaction: From Clear Solutions to a Cloudy Mixture
Visually, the reaction is quite striking. Mixing clear, colorless aqueous solutions of barium chloride and sodium sulphate results in the immediate formation of a white, cloudy precipitate. This precipitate, barium sulphate, gradually settles to the bottom of the container, leaving behind a relatively clear solution of sodium chloride. The change in appearance is a direct consequence of the formation of the insoluble barium sulphate solid. The intensity of the cloudiness is directly related to the concentrations of the reactants. Higher concentrations will lead to a more significant amount of precipitate, resulting in a denser, whiter cloud.
Microscopic View of Precipitate Formation
At a microscopic level, the formation of the precipitate involves the aggregation of barium and sulphate ions. Initially, the ions are randomly dispersed in the solution. Upon mixing, the electrostatic attraction between the oppositely charged ions overcomes the thermal motion, leading to the formation of small barium sulphate particles. These particles then collide and aggregate, growing in size until they become visible as the precipitate.
Stoichiometry and Calculations: Quantifying the Reaction
The balanced chemical equation allows us to perform stoichiometric calculations, determining the amounts of reactants needed to produce a specific amount of product or vice versa. For instance, we can calculate the theoretical yield of barium sulphate given the amounts of barium chloride and sodium sulphate used. Similarly, we can determine the limiting reactant if the amounts of the reactants are not stoichiometrically equivalent. These calculations are essential in quantitative analysis and experimental design.
Applications of the Reaction: Beyond the Classroom
While often used as a demonstrative experiment in chemistry classrooms, the reaction between barium chloride and sodium sulphate holds several practical applications:
1. Gravimetric Analysis: Precisely Measuring Quantities
The insolubility of barium sulphate makes this reaction a cornerstone of gravimetric analysis. In this analytical technique, the precise mass of the barium sulphate precipitate is used to determine the concentration of either barium or sulphate ions in an unknown sample. This method is highly accurate and widely employed in various analytical chemistry settings.
2. Wastewater Treatment: Removing Harmful Ions
Barium sulphate precipitation can be utilized in wastewater treatment to remove harmful sulphate ions. Adding a soluble barium salt to wastewater containing sulphate ions leads to the precipitation of barium sulphate, effectively removing the sulphate from the water. The precipitated barium sulphate can then be separated from the water through filtration or other separation techniques. However, due to the toxicity of barium compounds, careful consideration of environmental impact is essential.
3. Medical Applications (Contrast Agents): Visualizing Internal Structures
Barium sulphate, due to its high density and radiopacity, finds crucial application in medical imaging as a contrast agent. It's used in barium meals and barium enemas to visualize the gastrointestinal tract during X-ray examinations. The high atomic number of barium enhances the contrast between the digestive tract and surrounding tissues, enabling better visualization of abnormalities.
4. Pigment Production: Creating White Colors
In the past, barium sulphate, also known as blanc fixe, was used as a pigment in paints and coatings, providing a bright white color. However, its use has decreased due to the availability of more cost-effective alternatives and environmental concerns related to barium compounds.
Safety Precautions: Handling Chemicals Responsibly
It's crucial to emphasize the importance of safety when handling the chemicals involved in this reaction. Both barium chloride and barium sulphate are toxic if ingested, and precautions must be taken to prevent accidental ingestion or inhalation. Appropriate personal protective equipment (PPE), including safety goggles and gloves, should be worn throughout the experiment. Proper disposal of the waste products is also critical, following all relevant safety guidelines and regulations. The reaction should be conducted in a well-ventilated area.
Conclusion: A Simple Reaction with Profound Implications
The reaction between barium chloride and sodium sulphate, although seemingly simple, offers a rich tapestry of chemical principles and practical applications. From understanding solubility rules and stoichiometry to its use in gravimetric analysis and medical imaging, this reaction underscores the interconnectedness of different aspects of chemistry. However, the importance of safety cannot be overstated when working with these chemicals. By understanding the reaction mechanism, applying safe practices, and respecting environmental considerations, we can harness the power of this classic double displacement reaction effectively and responsibly. The careful study of this seemingly simple reaction provides a robust foundation for understanding more complex chemical processes and analytical techniques. Furthermore, the ability to predict and interpret the outcomes of this reaction reinforces the power of fundamental chemical principles in solving real-world problems.
Latest Posts
Latest Posts
-
What Is The Worlds Largest Gulf
Mar 20, 2025
-
What Is The Oxidation Number Of Phosphorus
Mar 20, 2025
-
Consumer Surplus Arises In A Market Because
Mar 20, 2025
-
Replace A B C By Suitable Numerals
Mar 20, 2025
-
Land Is Considered A Resource Because It
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Barium Chloride And Sodium Sulphate Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
