Write The Distribution Of Electrons In Carbon And Sodium Atoms
News Leon
Mar 20, 2025 · 5 min read
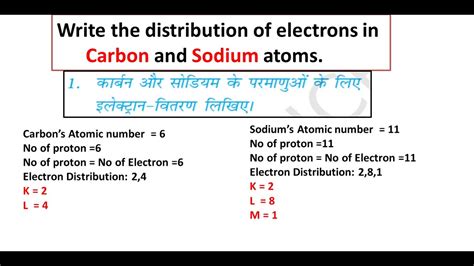
Table of Contents
Electron Distribution in Carbon and Sodium Atoms: A Deep Dive
Understanding the distribution of electrons within an atom is fundamental to comprehending its chemical behavior and properties. This article delves into the electron configurations of two crucial elements – carbon and sodium – exploring their atomic structures, the principles governing electron placement, and the implications of these arrangements for their reactivity and applications.
The Quantum Mechanical Model: Understanding Electron Shells and Subshells
Before diving into the specifics of carbon and sodium, let's establish the foundational framework. Electrons don't orbit the nucleus in neat, predictable paths like planets around a star. Instead, the quantum mechanical model describes electron behavior probabilistically. Electrons occupy regions of space called orbitals, which are defined by specific energy levels and shapes.
These orbitals are grouped into shells (also known as energy levels), designated by principal quantum numbers (n = 1, 2, 3...). Within each shell, there are subshells, characterized by their shapes and designated by the azimuthal quantum number (l = 0, 1, 2... n-1). These subshells are further categorized as:
- s subshells: Spherical in shape, holding a maximum of 2 electrons.
- p subshells: Dumbbell-shaped, holding a maximum of 6 electrons (three orbitals, each holding 2 electrons).
- d subshells: More complex shapes, holding a maximum of 10 electrons.
- f subshells: Even more complex shapes, holding a maximum of 14 electrons.
The Aufbau principle dictates that electrons first fill the lowest energy levels available. The Pauli exclusion principle states that each orbital can hold a maximum of two electrons with opposite spins. Finally, Hund's rule suggests that electrons will individually occupy orbitals within a subshell before pairing up. These principles guide us in determining the electron configuration of any atom.
Electron Configuration of Carbon (Atomic Number 6)
Carbon, a cornerstone element of organic chemistry, possesses six electrons. Applying the Aufbau principle, Pauli exclusion principle, and Hund's rule, we arrive at its electron configuration:
1s² 2s² 2p²
Let's break this down:
- 1s²: Two electrons fill the lowest energy level, the 1s subshell.
- 2s²: Two electrons fill the 2s subshell.
- 2p²: Two electrons occupy the 2p subshell. Following Hund's rule, these two electrons will occupy separate 2p orbitals before pairing up in the same orbital.
This configuration signifies that carbon has two electrons in its innermost shell (n=1) and four electrons in its outermost shell (n=2), also known as the valence shell. These four valence electrons are crucial in determining carbon's tetravalency – its ability to form four covalent bonds, underpinning its remarkable ability to form long chains and complex structures, forming the backbone of all organic molecules.
Visualizing Carbon's Electron Configuration:
Imagine the nucleus at the center. Around it, the first shell (n=1) contains a single spherical s orbital with two electrons. The second shell (n=2) consists of a spherical s orbital (containing two electrons) and three dumbbell-shaped p orbitals (containing two electrons, one in each orbital initially, following Hund's rule).
Electron Configuration of Sodium (Atomic Number 11)
Sodium, an alkali metal, has eleven electrons. Its electron configuration is:
1s² 2s² 2p⁶ 3s¹
- 1s²: The first shell contains two electrons.
- 2s²: The second shell contains two electrons in the s subshell.
- 2p⁶: The second shell also contains a full p subshell with six electrons.
- 3s¹: The third shell contains a single electron in the s subshell.
Unlike carbon, sodium's outermost shell (n=3) contains only one electron. This single valence electron is easily lost, making sodium highly reactive and readily forming a +1 cation (Na⁺). This explains sodium's characteristic properties, including its low ionization energy and high reactivity.
Visualizing Sodium's Electron Configuration:
Sodium's atomic structure is similar to carbon, but with additional shells. It has a filled first shell (1s²), a filled second shell (2s²2p⁶), and a third shell containing only one electron in its 3s orbital. This single electron in the outermost shell is responsible for sodium's reactivity.
Comparing Carbon and Sodium: Implications of Electron Configuration
The differences in their electron configurations directly impact the chemical properties and behaviors of carbon and sodium:
-
Reactivity: Sodium, with its single valence electron, is highly reactive, readily losing that electron to achieve a stable octet (eight electrons in its outermost shell). Carbon, with four valence electrons, is less reactive but can form strong covalent bonds by sharing electrons to achieve a stable octet.
-
Bonding: Sodium primarily forms ionic bonds by transferring its valence electron to another atom, resulting in the formation of ionic compounds. Carbon primarily forms covalent bonds by sharing electrons with other atoms, resulting in the formation of a vast array of organic compounds.
-
Electrical Conductivity: Sodium, as a metal, conducts electricity well because its loosely held valence electron can easily move. Carbon, in its pure diamond form, is an insulator; however, in its graphite form (with its layered structure), it can conduct electricity.
-
Applications: The unique properties of carbon and sodium lead to a wide range of applications. Carbon is essential in various forms (diamond, graphite, fullerenes) for applications in electronics, materials science, and medicine. Sodium is crucial in various biological processes and is used in various industrial applications, including the production of sodium hydroxide (lye) and sodium lamps.
Further Considerations: Excited States and Orbitals Shapes
The electron configurations discussed above represent the ground states of carbon and sodium atoms. When energy is absorbed, an electron can jump to a higher energy level, resulting in an excited state. These excited states are temporary and the electron eventually returns to its ground state, often releasing energy as light.
Furthermore, while we've simplified the description of orbital shapes, the true shapes are more complex, influenced by the interactions between electrons within the atom. Sophisticated techniques are used to visualize and understand the precise electron density distribution within atoms.
Conclusion: The Significance of Electron Configuration
The distribution of electrons within an atom profoundly influences its chemical properties and reactivity. Understanding the electron configurations of elements like carbon and sodium allows us to predict their bonding behavior, understand their reactivity, and explain their diverse applications in various fields. The principles outlined in this article—the Aufbau principle, Pauli exclusion principle, and Hund's rule—provide a powerful framework for understanding the electron arrangement of all elements in the periodic table, laying the foundation for a deeper understanding of chemistry and material science. The ability to visualize and interpret electron configurations is a vital skill for anyone studying chemistry or related disciplines.
Latest Posts
Latest Posts
-
Which Of The Following Combinations Are Correctly Matched
Mar 28, 2025
-
The Figure Gives An Overhead View Of The Path
Mar 28, 2025
-
A Galvanometer Has A Resistance Of 20 Ohm
Mar 28, 2025
-
What Are The Coordinates Of Point Q
Mar 28, 2025
-
30 As A Product Of Prime Factors
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Write The Distribution Of Electrons In Carbon And Sodium Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
