Why Is Hydrogen In Group 1
News Leon
Mar 21, 2025 · 5 min read
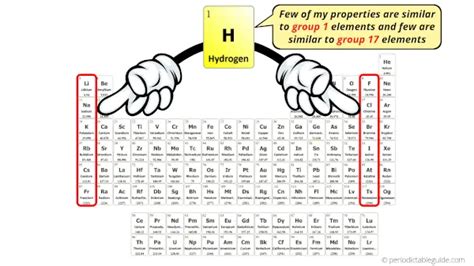
Table of Contents
Why is Hydrogen in Group 1? A Deep Dive into the Periodic Table's Enigma
Hydrogen, the simplest and most abundant element in the universe, occupies a unique and often debated position in the periodic table. While commonly placed in Group 1 (alkali metals) due to its single valence electron, its properties significantly differ from those of its alkali metal neighbors. This article delves into the reasoning behind its Group 1 placement, explores its anomalous behavior, and examines alternative classification schemes.
The Electron Configuration Argument: Why Group 1?
The primary justification for placing hydrogen in Group 1 stems from its electronic configuration. Like lithium, sodium, potassium, and other alkali metals, hydrogen possesses a single electron in its outermost shell (1s¹). This configuration dictates its potential to lose this electron and form a +1 cation, similar to the alkali metals. This shared characteristic forms the cornerstone of its traditional Group 1 placement.
Valence Electron Similarity: A Key Factor
The presence of a single valence electron is paramount. This electron is readily available for bonding, leading to the formation of ionic compounds with non-metals. For example, hydrogen readily reacts with halogens to form hydrogen halides (HCl, HBr, HF, HI), mirroring the behavior of alkali metals forming ionic salts. This reactive similarity with alkali metals provides further support for its Group 1 placement.
Ionic Bonding Parallels: Supporting the Argument
The formation of ionic compounds with a +1 charge further reinforces hydrogen's resemblance to alkali metals. While the hydrogen ion (H⁺) is significantly smaller and more reactive than alkali metal ions, the fundamental principle of ionic bonding remains the same. This similarity in bonding behavior, however subtle, contributes to the justification for its inclusion in Group 1.
The Anomalous Behavior: Where Hydrogen Diverges
Despite the electronic configuration similarity, hydrogen demonstrates significant deviations from the characteristic properties of alkali metals. These differences warrant careful consideration when discussing its placement within the periodic table.
Non-Metallic Properties: A Stark Contrast
Unlike alkali metals, hydrogen exists primarily as a diatomic gas (H₂) under standard conditions. This contrasts sharply with the metallic solid state of alkali metals. Furthermore, hydrogen exhibits non-metallic properties, such as poor electrical and thermal conductivity. Alkali metals, on the other hand, are excellent conductors. This significant difference in physical properties questions its inclusion in a group primarily characterized by metallic elements.
Covalent Bonding Predominance: A Unique Characteristic
While capable of ionic bonding, hydrogen more frequently forms covalent bonds. This tendency arises from its high electronegativity compared to alkali metals. Covalent bonding involves the sharing of electrons, a mechanism fundamentally different from the electron transfer observed in ionic bonding characteristic of alkali metals. This contrasting bonding preference underscores hydrogen's distinct nature.
Reactivity Differences: More than just a Single Electron
While both hydrogen and alkali metals readily react, the nature and products of their reactions often differ significantly. For instance, hydrogen reacts explosively with oxygen to form water, a reaction vastly different from the relatively less reactive alkali metals. The differences in reaction kinetics and products highlight the nuances of hydrogen's reactivity compared to its Group 1 counterparts.
Alternative Classification Schemes: Exploring Other Options
Given the inconsistencies in its properties relative to alkali metals, alternative classification schemes have been proposed for hydrogen. These alternatives aim to better reflect its unique chemical and physical behavior.
Placement Above the Halogens: An Appealing Alternative
Some argue for placing hydrogen above the halogens (Group 17) in the periodic table. This alternative stems from hydrogen's ability to gain an electron to achieve a stable noble gas configuration (He), forming a hydride ion (H⁻). This behavior mirrors the halogens' tendency to gain an electron to achieve a stable configuration. This perspective provides an alternative classification that accounts for hydrogen's ability to form negative ions.
A Unique Standalone Position: Acknowledging its Uniqueness
Another perspective suggests that hydrogen should occupy a unique position outside the main group classifications. This proposal acknowledges hydrogen's distinct properties and avoids forcing it into a group where it doesn't fully conform. This classification recognizes hydrogen's unique position in the chemical landscape without attempting to force it into a pre-defined group.
Bridging the Gap: A Compromise Approach
Some chemists propose a compromise approach: placing hydrogen in both Group 1 and Group 17, highlighting its duality. This acknowledges its ability to behave like both alkali metals and halogens, although rarely simultaneously. This bridging approach allows for the inclusion of hydrogen in both groups, effectively reflecting its diverse bonding behavior.
The Ongoing Debate: A Matter of Perspective
The debate surrounding hydrogen's placement remains a fascinating topic in chemistry. There's no single universally accepted solution. The choice of placement often reflects the emphasis on particular properties or bonding characteristics. It highlights the complexities of the periodic table and the limitations of a simple, one-dimensional classification system.
Pedagogical Considerations: A Teaching Tool
The ongoing debate also serves as a valuable pedagogical tool. It forces students to critically examine the properties of elements and the limitations of classification schemes. It emphasizes that periodic trends are not absolute and that exceptions exist.
Research Implications: Further Understanding
The nuances of hydrogen's properties continue to drive research. Understanding its unique behavior is critical in diverse fields, such as fuel cell technology, materials science, and astrophysics. The exploration of hydrogen's unique characteristics fosters deeper understanding of fundamental chemical principles.
Conclusion: A Complex Element in a Complex Table
Hydrogen's unique position in the periodic table underscores the limitations of a strictly defined classification system. While its single valence electron justifies its traditional placement in Group 1, its non-metallic properties, covalent bonding preference, and ability to form hydrides clearly differentiate it from the alkali metals. The ongoing debate over its classification reflects the richness and complexity of the chemical world and provides a valuable learning opportunity for students and researchers alike. Further research and innovative classification schemes may eventually provide a more definitive solution, but for now, hydrogen's position remains a testament to the complexities and nuances of the periodic table.
Latest Posts
Latest Posts
-
Do Covalent Compounds Dissolve In Water
Mar 27, 2025
-
What Is The Specific Heat Capacity Of Aluminium
Mar 27, 2025
-
Do Noble Gases Have Ionization Energy
Mar 27, 2025
-
How To Write An Invitation Letter To A Friend
Mar 27, 2025
-
The System In The Figure Below Is In Equilibrium
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Why Is Hydrogen In Group 1 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
