Why Does Atomic Radius Decrease From Left To Right
News Leon
Mar 29, 2025 · 5 min read
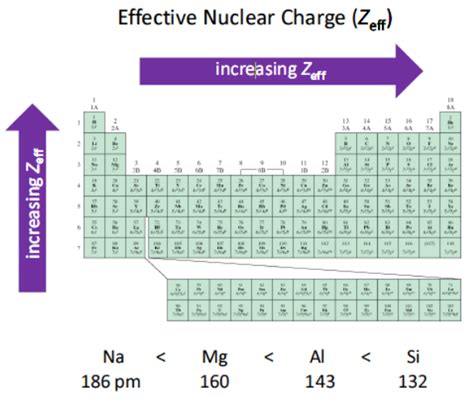
Table of Contents
Why Does Atomic Radius Decrease From Left to Right Across a Period?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. One crucial trend observed is the decrease in atomic radius from left to right across a period (row). This seemingly simple observation stems from complex interplay of fundamental forces within the atom. Understanding this trend requires a grasp of concepts like effective nuclear charge, shielding effect, and electron-electron repulsion. This article delves deep into the reasons behind this periodic trend, explaining it in a clear, concise, and comprehensive manner.
Understanding Atomic Radius
Before we dive into the reasons for the decrease, let's define atomic radius. Atomic radius refers to the distance from the nucleus of an atom to its outermost electron. It's important to note that this isn't a fixed, easily measurable quantity. It’s often determined indirectly through various methods, such as X-ray crystallography or spectroscopic techniques, which provide estimates of the average distance. Different methods may yield slightly different values, but the overall trend remains consistent.
The Role of Effective Nuclear Charge (Z<sub>eff</sub>)
The primary driver behind the decrease in atomic radius across a period is the increase in effective nuclear charge (Z<sub>eff</sub>). Z<sub>eff</sub> represents the net positive charge experienced by an electron in a multi-electron atom. It's not simply the total positive charge of the nucleus (the number of protons, Z), but rather the charge felt after accounting for the shielding effect of inner electrons.
Shielding Effect: Inner Electrons' Protective Layer
Inner electrons, those residing in energy levels closer to the nucleus, partially shield outer electrons from the full positive charge of the nucleus. This shielding effect reduces the attractive force experienced by outer electrons. The more inner electrons present, the greater the shielding.
Increasing Z<sub>eff</sub> Across a Period: The Nucleus's Stronger Pull
As we move from left to right across a period, the number of protons in the nucleus increases. While additional electrons are also added (to maintain electrical neutrality), these electrons are added to the same principal energy level (or shell). The increase in nuclear charge outweighs the increase in shielding provided by the newly added electrons. This means that Z<sub>eff</sub> increases significantly. The stronger positive charge from the nucleus pulls the outer electrons closer, thus reducing the atomic radius.
Electron-Electron Repulsion: A Counteracting Force
While the increase in Z<sub>eff</sub> is the dominant factor, it's important to acknowledge the role of electron-electron repulsion. As more electrons are added to the same energy level, the repulsion between these negatively charged particles increases. This repulsion pushes the electrons slightly further apart, counteracting the inward pull of the increased Z<sub>eff</sub>.
However, the effect of electron-electron repulsion is relatively minor compared to the effect of the increasing Z<sub>eff</sub>. The increase in nuclear charge dominates, resulting in a net decrease in atomic radius. This is why the atomic radius generally decreases across a period, despite the increasing electron-electron repulsion.
Illustrative Examples: Comparing Atomic Radii
Let's consider the second period (Li to Ne) as an example:
- Lithium (Li): Has 3 protons and 2 inner electrons shielding the single outer electron. Z<sub>eff</sub> is relatively low.
- Beryllium (Be): Has 4 protons and 2 inner electrons shielding 2 outer electrons. Z<sub>eff</sub> is higher than Li.
- Boron (B): Has 5 protons and 2 inner electrons shielding 3 outer electrons. Z<sub>eff</sub> is even higher.
This trend continues across the period, with each subsequent element exhibiting a higher Z<sub>eff</sub> and therefore a smaller atomic radius. By the time we reach Neon (Ne), the highest Z<sub>eff</sub> in the period results in the smallest atomic radius.
Exceptions and Nuances
While the general trend of decreasing atomic radius across a period is well-established, there can be minor irregularities. These exceptions are often subtle and usually attributed to electron-electron repulsion effects, specifically in the case of some p-block elements. For instance, a slightly larger atomic radius might be observed where the addition of an electron initiates a new sublevel or half-fills a sublevel, causing increased electron-electron repulsion.
However, these deviations are generally small and do not negate the overall trend of decreasing atomic radius from left to right.
Connecting Atomic Radius to Other Properties
The decrease in atomic radius across a period has significant implications for other atomic properties. For example:
- Ionization Energy: As atomic radius decreases, the outermost electrons are held more tightly by the nucleus. This leads to a higher ionization energy, the energy required to remove an electron.
- Electronegativity: The stronger pull of the nucleus on outer electrons also increases electronegativity, the ability of an atom to attract electrons in a chemical bond.
- Electron Affinity: This refers to the energy change when an electron is added to a neutral atom. A smaller atomic radius usually correlates with a higher electron affinity, as the incoming electron experiences a stronger attraction from the nucleus.
Conclusion: A Fundamental Periodic Trend
The decrease in atomic radius across a period is a fundamental and crucial periodic trend in chemistry. It directly arises from the interplay between the increasing effective nuclear charge and the relatively less influential electron-electron repulsion. This seemingly simple trend has far-reaching consequences, affecting various other atomic properties and ultimately influencing the chemical behavior of elements. Understanding this trend is key to comprehending the organization and properties of elements within the periodic table.
Keywords:
Atomic radius, effective nuclear charge (Z<sub>eff</sub>), shielding effect, electron-electron repulsion, periodic table, ionization energy, electronegativity, electron affinity, periodic trends, chemical properties, atomic structure, protons, electrons, nucleus, valence electrons, principal energy levels, sublevels, orbitals.
Related Terms:
Ionic radius, covalent radius, van der Waals radius, isoelectronic series, periodicity, chemical reactivity.
Latest Posts
Latest Posts
-
Why Is The Testes Located Outside The Body
Mar 31, 2025
-
Which Of The Following Has The Smallest Radius
Mar 31, 2025
-
What Is The Atomic Mass Of Strontium
Mar 31, 2025
-
How Is Evaporation Different From Boiling
Mar 31, 2025
-
Mice Have 20 Bivalents Visible In Meiosis I
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Why Does Atomic Radius Decrease From Left To Right . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
