What Is The Atomic Mass Of Strontium
News Leon
Mar 31, 2025 · 6 min read
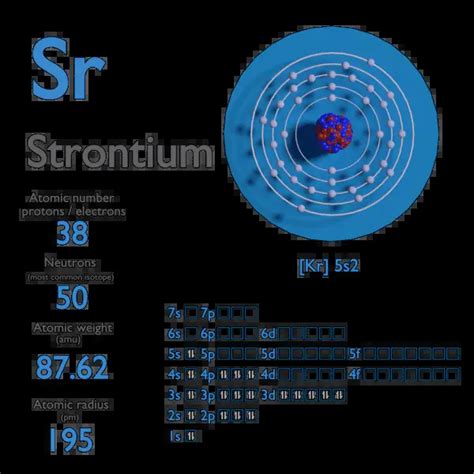
Table of Contents
What is the Atomic Mass of Strontium? A Deep Dive into Isotopes and Applications
Strontium, a fascinating alkaline earth metal, finds itself nestled within the periodic table, gracing us with its presence in Group 2, Period 5. Its chemical symbol, Sr, immediately tells us it’s a player in the world of chemistry and beyond. But beyond its position, one of the most fundamental properties of strontium is its atomic mass. Understanding this seemingly simple number requires delving into the intricacies of isotopes and their applications. This comprehensive article will not only answer the question "What is the atomic mass of strontium?" but also explore the underlying science and its significance.
Understanding Atomic Mass: More Than Just a Number
The atomic mass of an element isn't a single, fixed value but rather a weighted average of the masses of all its naturally occurring isotopes. An isotope is an atom of the same element that possesses the same number of protons but differs in the number of neutrons in its nucleus. This difference in neutron count directly impacts the atom's mass. Since neutrons contribute significantly to an atom's mass (along with protons), different isotopes of the same element will have different masses.
Think of it like this: imagine you're calculating the average weight of a group of people. Some are tall and heavy, some are short and light. The average weight doesn't represent the weight of any single individual, but rather, a weighted average considering the number of people in each weight category. Atomic mass operates on a similar principle.
Strontium's Isotopic Composition: The Key to its Atomic Mass
Strontium boasts four stable isotopes found in nature: ⁸⁴Sr, ⁸⁶Sr, ⁸⁷Sr, and ⁸⁸Sr. Each isotope contributes to the overall atomic mass, with its contribution weighted by its relative abundance. The relative abundance refers to the percentage of each isotope present in a naturally occurring sample of strontium. These abundances aren't completely uniform across all strontium sources; slight variations can exist depending on geological location. However, generally accepted average abundances are used to calculate the standard atomic weight.
- ⁸⁴Sr: This isotope has a relatively low abundance and therefore contributes less significantly to the weighted average atomic mass.
- ⁸⁶Sr: Possessing a moderate abundance, ⁸⁶Sr plays a more substantial role in determining strontium's average atomic mass.
- ⁸⁷Sr: This isotope stands out due to its unique origin and geological applications, which we'll discuss later.
- ⁸⁸Sr: This is the most abundant isotope of strontium and consequently holds the most significant weight in calculating the average atomic mass.
Calculating the Atomic Mass of Strontium: A Step-by-Step Approach
Calculating the atomic mass of strontium requires knowing the mass and relative abundance of each stable isotope. The calculation is a weighted average:
Atomic Mass = (Mass of isotope 1 × Abundance of isotope 1) + (Mass of isotope 2 × Abundance of isotope 2) + ...
Let's assume the following abundances (these may vary slightly depending on the source):
- ⁸⁴Sr (83.9134 amu): 0.56%
- ⁸⁶Sr (85.9093 amu): 9.86%
- ⁸⁷Sr (86.9089 amu): 7.00%
- ⁸⁸Sr (87.9056 amu): 82.58%
Using these values, the calculation would be:
(83.9134 amu × 0.0056) + (85.9093 amu × 0.0986) + (86.9089 amu × 0.0700) + (87.9056 amu × 0.8258) ≈ 87.62 amu
Therefore, the average atomic mass of strontium is approximately 87.62 atomic mass units (amu). Note that this is an average; individual strontium atoms will have a mass corresponding to one of its isotopes. The slight variations in reported atomic mass values across different sources stem from variations in the measured isotopic abundances.
The Significance of Strontium's Isotopic Composition: Beyond the Atomic Mass
The isotopic composition of strontium isn't just relevant for calculating its atomic mass; it holds significant importance in various scientific fields:
1. Geochronology and Geology: Tracing Earth's History
The abundance of ⁸⁷Sr, specifically, is crucial in geochronology, the science of dating rocks and minerals. ⁸⁷Sr is a radiogenic isotope, meaning it's formed by the radioactive decay of ⁸⁷Rb (Rubidium-87). By analyzing the ⁸⁷Sr/⁸⁶Sr ratio in rocks, geologists can determine the age of the rock and even gain insights into the geological processes that shaped it. This technique is invaluable for understanding the Earth's history and evolution.
2. Archaeology and Anthropology: Tracking Human Migrations and Diet
The ⁸⁷Sr/⁸⁶Sr ratio in human teeth and bones reflects the isotopic composition of the strontium in the environment where an individual grew up. This "strontium signature" can be used in archaeology and anthropology to trace human migrations and understand ancient diets. Differences in the ⁸⁷Sr/⁸⁶Sr ratio between an individual's bones and the environment where their remains were found can indicate migration patterns.
3. Environmental Science: Monitoring Pollution and Tracing Contaminants
Strontium isotopes can also be used in environmental science to track pollution and trace contaminants. The isotopic signature of strontium in different environmental sources can provide insights into the origin and spread of pollutants.
Applications of Strontium: From Fireworks to Medicine
The unique properties of strontium extend beyond its isotopic composition. It finds a wide range of applications in various industries:
1. Fireworks: Adding Brilliant Red Color
Strontium compounds are commonly used in fireworks to produce a vibrant red color. The intense red glow is due to the electronic transitions within strontium atoms when they are excited by heat.
2. Metallurgy: Alloying and Refining
Strontium is used in metallurgy to refine metals and create alloys with specific properties. It can enhance the strength and ductility of certain metals.
3. Medicine: Treating Bone Diseases
Certain strontium compounds are used in medicine, particularly for treating bone diseases like osteoporosis. Strontium ranelate, for example, can increase bone density and reduce the risk of fractures.
4. Electronics: Components and Devices
Strontium's properties make it useful in some electronic components and devices. For instance, it's found in certain types of ferrites and ceramic materials.
Conclusion: The Atomic Mass of Strontium and its Broader Significance
The atomic mass of strontium, approximately 87.62 amu, is a weighted average reflecting the relative abundances of its four stable isotopes. However, the significance of strontium extends far beyond this simple number. Its isotopic composition plays a crucial role in various scientific fields, from geochronology to archaeology and environmental science. Moreover, strontium’s unique properties lead to diverse applications ranging from fireworks to medicine, showcasing its importance across various sectors. Understanding the atomic mass and isotopic variations of strontium is key to unlocking a deeper understanding of its applications and its role in the natural world. Further research into strontium's isotopes continues to refine our understanding of Earth's history, human migration, and environmental processes, demonstrating the ongoing importance of this remarkable element.
Latest Posts
Latest Posts
-
What Is The Difference Between A Primary And Secondary Consumer
Apr 02, 2025
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Is The Atomic Mass Of Strontium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
