How Is Evaporation Different From Boiling
News Leon
Mar 31, 2025 · 5 min read
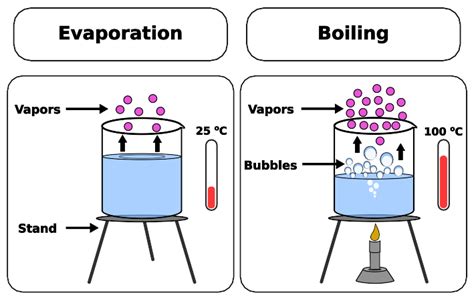
Table of Contents
How is Evaporation Different from Boiling? Understanding the Subtleties of Liquid-to-Gas Transitions
Evaporation and boiling might seem like interchangeable terms, both describing the transition of a liquid into a gas. However, a closer look reveals significant differences in the mechanisms, conditions, and observable characteristics of these two processes. Understanding these nuances is crucial in various scientific fields, from meteorology and climatology to chemical engineering and everyday cooking. This comprehensive guide delves into the core differences between evaporation and boiling, exploring the underlying physics and providing practical examples to solidify your understanding.
The Essence of Evaporation: A Surface Phenomenon
Evaporation is a surface phenomenon, meaning it occurs only at the surface of a liquid. Molecules within the liquid are constantly in motion, colliding with each other and transferring energy. Some molecules near the surface possess sufficient kinetic energy to overcome the intermolecular forces holding them in the liquid phase. These high-energy molecules escape the liquid's surface and transition into the gaseous phase, becoming vapor.
Factors Influencing Evaporation Rate:
Several factors significantly influence the rate of evaporation:
-
Temperature: Higher temperatures translate to increased kinetic energy of liquid molecules, leading to a faster evaporation rate. More molecules possess the energy needed to escape the liquid's surface.
-
Surface Area: A larger surface area exposes more liquid molecules to the surrounding environment, accelerating the evaporation process. This is why spreading out a liquid increases its evaporation rate.
-
Humidity: Humidity, or the amount of water vapor already present in the air, plays a crucial role. If the air is saturated with water vapor, the rate of evaporation slows significantly as fewer molecules can escape into the already crowded gaseous phase. Dry air, conversely, promotes faster evaporation.
-
Air Movement (Wind): Wind or any air movement sweeps away the water vapor molecules near the liquid's surface, reducing the concentration of water vapor in the immediate vicinity. This creates a concentration gradient, encouraging more molecules to evaporate to maintain equilibrium.
-
Type of Liquid: Different liquids have different intermolecular forces. Liquids with weaker intermolecular forces, such as volatile organic compounds, evaporate more quickly than those with stronger forces, like water.
Think of it this way: Imagine a crowded room. People (liquid molecules) are constantly moving. Those near the door (surface) with enough energy might push their way out (evaporate). If the room is already crowded outside (high humidity), it's harder to escape. If there's a breeze (wind) pulling people out, more can leave.
Boiling: A Bulk Phenomenon at a Specific Temperature
Boiling, on the other hand, is a bulk phenomenon that occurs throughout the entire volume of the liquid. It's characterized by the formation of vapor bubbles within the liquid itself, not just at the surface. This dramatic process only occurs when the liquid reaches its boiling point.
The Boiling Point: A Critical Temperature
The boiling point is the temperature at which the vapor pressure of the liquid equals the external pressure exerted on the liquid. At this point, the liquid's molecules have enough kinetic energy to overcome the intermolecular forces and the external pressure, allowing vapor bubbles to form and rise to the surface.
Factors Affecting Boiling Point:
Several factors influence the boiling point:
-
External Pressure: At higher external pressures, the boiling point increases, as the liquid needs to overcome a greater pressure to form vapor bubbles. Conversely, at lower pressures, the boiling point decreases. This is why water boils at a lower temperature at high altitudes where atmospheric pressure is lower.
-
Impurities: The presence of dissolved impurities in a liquid can slightly elevate its boiling point. This is known as boiling point elevation.
-
Type of Liquid: Different liquids have different boiling points, reflecting the strength of their intermolecular forces. Liquids with stronger intermolecular forces require higher temperatures to reach their boiling points.
Imagine it like this: A pot of water on the stove. As you heat it, the molecules gain energy. At the boiling point, the energy is sufficient to create bubbles of steam within the water, not just at the surface. These bubbles rise and burst at the surface.
Key Differences Summarized:
| Feature | Evaporation | Boiling |
|---|---|---|
| Location | Surface only | Throughout the liquid volume |
| Temperature | Occurs at any temperature below boiling | Occurs at the boiling point only |
| Mechanism | Individual molecules escape surface | Vapor bubbles form and rise to the surface |
| Pressure | Independent of external pressure | Dependent on external pressure |
| Rate | Variable, affected by many factors | Constant at a given pressure |
| Visibility | Often subtle, not easily observed | Easily visible, vigorous process |
Real-World Applications and Examples:
Understanding the differences between evaporation and boiling is crucial in various applications:
-
Weather Forecasting: Evaporation plays a vital role in the water cycle, influencing humidity levels and cloud formation. Boiling is less relevant in this context.
-
Cooking: Both processes are important in cooking. Evaporation reduces liquid volume during simmering or reducing sauces, while boiling is used for cooking pasta or vegetables.
-
Chemical Engineering: Evaporation and distillation (a process utilizing both evaporation and condensation) are essential techniques for separating mixtures. Boiling point differences allow for separating components of mixtures.
-
Refrigeration: Evaporation of refrigerants absorbs heat, a principle used in refrigerators and air conditioners.
-
Desalination: Evaporation is a common method for desalination, separating salt from seawater to obtain fresh water.
Further Exploration: Sublimation and Deposition
Beyond evaporation and boiling, other phase transitions exist. Sublimation is the transition of a solid directly into a gas, bypassing the liquid phase (e.g., dry ice). Deposition is the reverse process, where a gas directly transitions into a solid (e.g., frost formation). These processes, while different from evaporation and boiling, highlight the multifaceted nature of phase transitions.
Conclusion: A Deeper Understanding of Liquid-to-Gas Transitions
Evaporation and boiling, while both involving liquid-to-gas transitions, are distinct processes with different mechanisms and characteristics. Evaporation is a surface phenomenon occurring at any temperature below the boiling point, while boiling is a bulk phenomenon occurring at the specific boiling point. Understanding these differences is essential in various scientific disciplines and everyday life, from meteorology and cooking to chemical engineering and refrigeration. This comprehensive overview has hopefully provided a clearer picture of these crucial liquid-to-gas transitions, equipping you with a deeper understanding of the fascinating world of phase changes.
Latest Posts
Latest Posts
-
Which One Of The Following Is An Igneous Rock
Apr 02, 2025
-
Which Is Greater 2 3 Or 3 5
Apr 02, 2025
-
Find The Area Of A Shaded Triangle
Apr 02, 2025
-
Which Of The Following Would Decrease Glomerular Filtration Rate
Apr 02, 2025
-
The Slope Of Speed Time Graph Indicates
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Is Evaporation Different From Boiling . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
