Which Set Of Compounds Illustrates The Law Of Multiple Proportions
News Leon
Mar 20, 2025 · 5 min read
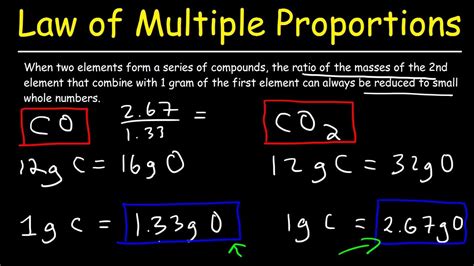
Table of Contents
Which Set of Compounds Illustrates the Law of Multiple Proportions?
The Law of Multiple Proportions, a cornerstone of chemistry, states that when two elements combine to form more than one compound, the different masses of one element that combine with a fixed mass of the other element are in a ratio of small whole numbers. This law, alongside the Law of Conservation of Mass and the Law of Definite Proportions, helped lay the foundation for Dalton's Atomic Theory. Understanding which sets of compounds best exemplify this law requires a careful examination of their chemical formulas and the ratios of the elements involved.
Understanding the Law of Multiple Proportions
Before diving into specific examples, let's reinforce the core concept. The law doesn't dictate that any two elements forming multiple compounds will always demonstrate this whole-number ratio. The crucial point is that when this relationship is observed, it supports the idea that matter is composed of discrete units – atoms – combining in specific, whole-number ratios. Fractional ratios would contradict this atomic theory.
Consider two elements, A and B. If they form two compounds, A<sub>x</sub>B<sub>y</sub> and A<sub>m</sub>B<sub>n</sub>, the law of multiple proportions states that the ratio of the mass of A that combines with a fixed mass of B in the two compounds will be a simple whole-number ratio (like 2:1, 3:2, etc.). This ratio is derived from the relative numbers of atoms of A and B in each compound.
Examples of Compounds Illustrating the Law
Several sets of compounds elegantly illustrate the Law of Multiple Proportions. Let's delve into some prominent examples:
1. Carbon Monoxide (CO) and Carbon Dioxide (CO₂)
This classic example is often the first introduced when teaching this fundamental chemical principle. Both carbon monoxide and carbon dioxide are binary compounds formed from the combination of carbon (C) and oxygen (O).
- Carbon Monoxide (CO): One carbon atom combines with one oxygen atom.
- Carbon Dioxide (CO₂): One carbon atom combines with two oxygen atoms.
Let's consider a fixed mass of carbon, say 12 grams (the molar mass of carbon).
- In CO, 12 grams of carbon combines with 16 grams of oxygen (molar mass of oxygen).
- In CO₂, 12 grams of carbon combines with 32 grams of oxygen.
The ratio of the masses of oxygen that combine with a fixed mass of carbon (12 grams) is 16:32, which simplifies to 1:2 – a simple whole-number ratio. This perfectly demonstrates the Law of Multiple Proportions.
2. Nitrogen Oxides (N<sub>x</sub>O<sub>y</sub>)
Nitrogen and oxygen form several oxides, providing a rich set of examples. Consider the following:
- Nitrous Oxide (N₂O): Two nitrogen atoms combine with one oxygen atom.
- Nitric Oxide (NO): One nitrogen atom combines with one oxygen atom.
- Nitrogen Dioxide (NO₂): One nitrogen atom combines with two oxygen atoms.
- Dinitrogen Tetroxide (N₂O₄): Two nitrogen atoms combine with four oxygen atoms.
- Dinitrogen Pentoxide (N₂O₅): Two nitrogen atoms combine with five oxygen atoms.
If we fix the mass of nitrogen, the masses of oxygen combining with it in these different compounds will exhibit a whole-number ratio. For instance, if we compare NO and NO₂, the ratio of oxygen masses combining with a fixed nitrogen mass is 1:2. Comparing N₂O and NO, the ratio becomes 1:2. The ratios consistently demonstrate the small whole-number relationship specified by the law.
3. Iron Oxides (Fe<sub>x</sub>O<sub>y</sub>)
Iron also forms multiple oxides, providing another compelling illustration.
- Iron(II) Oxide (FeO): One iron atom combines with one oxygen atom.
- Iron(III) Oxide (Fe₂O₃): Two iron atoms combine with three oxygen atoms.
Consider a fixed mass of iron. The ratio of oxygen masses combining with this fixed iron mass will again be a simple whole number ratio, directly reflecting the differing ratios of iron and oxygen atoms in the compounds.
4. Sulfur Oxides (S<sub>x</sub>O<sub>y</sub>)
Sulfur and oxygen also form multiple oxides, offering additional supporting evidence for the Law of Multiple Proportions. Compounds such as sulfur dioxide (SO₂) and sulfur trioxide (SO₃) clearly demonstrate this principle. The ratio of oxygen mass combining with a fixed sulfur mass is 2:3, a simple whole-number ratio.
Why Some Compound Sets Don't Illustrate the Law
It's crucial to understand that the Law of Multiple Proportions is not universally applicable to all compounds. It only applies to situations where two elements form multiple compounds. If only one compound is formed by two elements, the law doesn't come into play.
Furthermore, the law is specifically about the ratio of masses, not the absolute masses. It's about the relative proportions of the elements, which are dictated by their atomic ratios in the different compounds.
Applications and Significance of the Law
The Law of Multiple Proportions holds significant historical and practical value:
- Supporting Atomic Theory: It provided strong experimental support for Dalton's Atomic Theory, bolstering the concept of atoms combining in specific, whole-number ratios.
- Chemical Formula Determination: It aids in determining the chemical formulas of compounds, especially when dealing with multiple compounds of the same two elements. By analyzing the mass ratios, we can infer the relative numbers of atoms involved.
- Stoichiometry: Understanding this law is crucial for stoichiometric calculations, enabling precise predictions of reactant and product quantities in chemical reactions.
Conclusion
The Law of Multiple Proportions is a fundamental principle in chemistry that showcases the discrete nature of matter. While not universally applicable, when observed, it provides compelling evidence for the atomic theory and plays a vital role in various chemical calculations and analyses. The examples of carbon oxides, nitrogen oxides, iron oxides, and sulfur oxides demonstrate the clear and consistent relationship between the masses of elements in multiple compounds, reinforcing this critical law of chemical combination. Understanding these examples provides a firm grasp of this essential aspect of chemical science. Furthermore, it highlights how careful observation and analysis of experimental data can lead to significant breakthroughs in scientific understanding.
Latest Posts
Latest Posts
-
A Measure Of The Disorder Of A System Is Called
Mar 21, 2025
-
How To Separate Sugar And Water
Mar 21, 2025
-
Which Of The Following Is An Accessory Organ Of Digestion
Mar 21, 2025
-
Which Of The Following Is The Most Stable Radical
Mar 21, 2025
-
How To Balance H2o2 H2o O2
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which Set Of Compounds Illustrates The Law Of Multiple Proportions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
