How To Balance H2o2 H2o O2
News Leon
Mar 21, 2025 · 5 min read
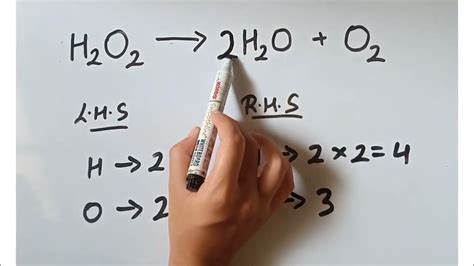
Table of Contents
How to Balance H₂O₂, H₂O, and O₂: A Comprehensive Guide to Chemical Equations
Balancing chemical equations is a fundamental concept in chemistry. It's crucial for understanding stoichiometry, predicting reaction outcomes, and performing accurate calculations. This in-depth guide focuses on balancing the equation involving hydrogen peroxide (H₂O₂), water (H₂O), and oxygen (O₂), a common decomposition reaction. We'll explore various methods, delve into the underlying principles, and provide practical examples to solidify your understanding.
Understanding the Decomposition of Hydrogen Peroxide
Hydrogen peroxide (H₂O₂) is an unstable compound that readily decomposes into water (H₂O) and oxygen (O₂). This decomposition is often catalyzed by various substances, such as manganese dioxide (MnO₂) or even light. The unbalanced equation representing this reaction is:
H₂O₂ → H₂O + O₂
This equation is unbalanced because the number of atoms of each element is not equal on both sides of the arrow. Balancing this equation requires adjusting the coefficients (the numbers placed in front of the chemical formulas) until the number of atoms of each element is the same on both the reactant (left) and product (right) sides.
Methods for Balancing Chemical Equations
Several methods can be used to balance chemical equations. Here, we'll explore two common and effective approaches:
1. The Inspection Method (Trial and Error)
This method involves systematically adjusting coefficients until the equation is balanced. It's a trial-and-error approach, but with practice, it becomes efficient. Let's apply it to the hydrogen peroxide decomposition:
-
Start with the most complex molecule: In this case, H₂O₂. We have two oxygen atoms on the reactant side.
-
Balance oxygen: On the product side, we have one oxygen atom in H₂O and two in O₂. To balance the oxygen atoms, we can place a coefficient of 2 in front of H₂O, giving us 2H₂O. This yields a total of four oxygen atoms on the product side.
-
Adjust the coefficient of H₂O₂: Now, to balance the oxygen, we need to place a coefficient of 2 in front of H₂O₂, giving us 2H₂O₂. This yields four oxygen atoms on the reactant side.
-
Check hydrogen: We now have four hydrogen atoms on both the reactant (2H₂O₂) and product (2H₂O) sides.
The balanced equation is:
2H₂O₂ → 2H₂O + O₂
This method works well for relatively simple equations. However, for more complex equations, it can become time-consuming and less efficient.
2. The Algebraic Method
This method utilizes algebraic equations to solve for the coefficients. It's particularly useful for complex reactions. Let's apply it to our example:
-
Assign variables: Assign variables to the coefficients:
aH₂O₂ → bH₂O + cO₂
-
Set up equations: Write equations based on the number of atoms of each element:
- Hydrogen: 2a = 2b
- Oxygen: 2a = b + 2c
-
Solve the equations: We have two equations and three unknowns. We can choose one variable and express the others in terms of it. Let's choose 'a' = 2.
-
Substituting 'a' = 2 into 2a = 2b, we get b = 2.
-
Substituting 'a' = 2 and b = 2 into 2a = b + 2c, we get 4 = 2 + 2c, which gives c = 1.
-
-
Write the balanced equation: Substitute the values of a, b, and c back into the equation:
2H₂O₂ → 2H₂O + O₂
This confirms that the balanced equation remains the same.
Beyond Balancing: Understanding the Reaction
The balanced equation 2H₂O₂ → 2H₂O + O₂ tells us much more than just the relative amounts of reactants and products. It provides quantitative information crucial for stoichiometric calculations.
-
Mole Ratios: The coefficients represent the mole ratios of the reactants and products. For every 2 moles of hydrogen peroxide that decompose, 2 moles of water and 1 mole of oxygen are produced.
-
Mass Relationships: Using the molar masses of H₂O₂, H₂O, and O₂, we can calculate the mass relationships between the reactants and products. This allows for quantitative predictions of the amount of product formed from a given amount of reactant.
-
Reaction Rate: The rate of this decomposition reaction can be influenced by various factors, including temperature, catalysts, and concentration. Understanding these factors is important in controlling the reaction.
Practical Applications and Implications
The decomposition of hydrogen peroxide has numerous practical applications, including:
-
Disinfection: The released oxygen is a powerful oxidizing agent, making hydrogen peroxide an effective disinfectant in various settings, from medical applications to water treatment.
-
Rocket Propulsion: Hydrogen peroxide's decomposition releases significant energy, making it a potential fuel source for rockets.
-
Industrial Processes: Hydrogen peroxide is used as a bleaching agent in textile and paper industries.
Common Mistakes to Avoid
When balancing chemical equations, several common mistakes can lead to inaccurate results:
-
Ignoring Subscripts: Never change the subscripts within a chemical formula when balancing. This alters the chemical identity of the substance.
-
Incorrect Coefficient Placement: Coefficients must be placed before the entire chemical formula, not within it.
-
Not Checking the Balance: Always verify that the number of atoms of each element is equal on both sides after balancing.
-
Rushing the Process: Balancing equations requires careful attention to detail. Take your time to ensure accuracy.
Advanced Considerations
While the decomposition of hydrogen peroxide is a relatively straightforward reaction, understanding its nuances requires exploring additional concepts:
-
Reaction Kinetics: Studying the reaction rate and its dependence on factors like temperature and concentration provides a deeper understanding of the reaction mechanism.
-
Thermodynamics: Analyzing the enthalpy change (heat released or absorbed) during the reaction helps determine the reaction's spontaneity and energy balance.
-
Catalysis: Different catalysts can significantly alter the reaction rate, offering opportunities for controlling the reaction's speed and efficiency.
Conclusion
Balancing the chemical equation for the decomposition of hydrogen peroxide into water and oxygen is a fundamental skill in chemistry. Mastering the inspection and algebraic methods allows for accurate representation of chemical reactions and provides the foundation for understanding stoichiometry and reaction kinetics. By understanding the balanced equation and its implications, we can better grasp the significance of this reaction in diverse applications, from disinfection to industrial processes. Remember, practice is key. Work through various examples, and you'll develop proficiency in balancing chemical equations and comprehending their quantitative significance.
Latest Posts
Latest Posts
-
The System In The Figure Below Is In Equilibrium
Mar 27, 2025
-
Poisonous Substances Produced By Some Microorganisms Are Called
Mar 27, 2025
-
In What Cell Organelle Does Photosynthesis Occur
Mar 27, 2025
-
How Many Pi And Sigma Bonds In A Triple Bond
Mar 27, 2025
-
What Is The Difference Between A Community And Population
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Balance H2o2 H2o O2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
