Which Periodic Table Group Contains Only Metals
News Leon
Mar 24, 2025 · 4 min read
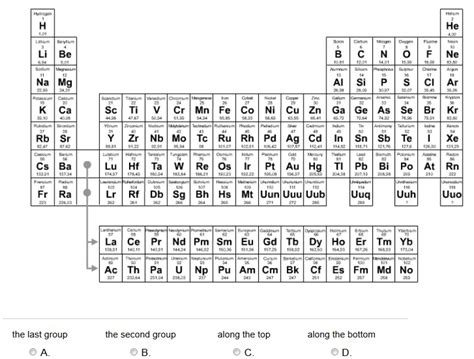
Table of Contents
- Which Periodic Table Group Contains Only Metals
- Table of Contents
- Which Periodic Table Group Contains Only Metals?
- Understanding the Periodic Table's Structure
- The Challenge of Defining "Metal"
- Group 3 (Scandium Family) and Beyond: A Closer Look
- The Lanthanides and Actinides: A Special Case
- The Importance of Context: Oxidation States and Allotropes
- Conclusion: No Single Group Contains Only Metals
- Latest Posts
- Latest Posts
- Related Post
Which Periodic Table Group Contains Only Metals?
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring chemical properties. Understanding its structure is crucial for predicting the behavior of elements and their compounds. One common question that arises, particularly for students beginning their chemistry journey, is: which group contains only metals? The answer isn't as straightforward as it might seem, requiring a deeper dive into the properties and classifications of elements.
Understanding the Periodic Table's Structure
The periodic table is arranged in rows (periods) and columns (groups or families). Elements within the same group share similar chemical properties due to having the same number of valence electrons—the electrons in the outermost shell. These valence electrons are primarily responsible for an element's reactivity and bonding characteristics. The groups are numbered from 1 to 18, though older numbering systems (IA, IIA, etc.) are sometimes still encountered.
Groups 1 (alkali metals) and 2 (alkaline earth metals) are well-known for containing highly reactive metals. However, the question of which group contains only metals necessitates a nuanced exploration. While several groups are predominantly metallic, achieving a group composed solely of metals presents some complexities.
The Challenge of Defining "Metal"
The simple definition of a metal as an element that is generally shiny, malleable, ductile, and a good conductor of heat and electricity is a useful starting point, but it's not entirely precise. Some elements exhibit properties that blur the lines between metals, non-metals, and metalloids (also called semi-metals). This ambiguity is especially pronounced in the transition metals and elements towards the border between metals and non-metals.
For example, while most elements in groups 3-12 (transition metals) are indeed metals, some display properties that deviate from the classic metal definition depending on their oxidation state or the specific conditions. Similarly, some elements at the edges of the periodic table, near the metalloid region, demonstrate properties that blend metallic and non-metallic characteristics.
Group 3 (Scandium Family) and Beyond: A Closer Look
Groups 3 through 12, the transition metals, are largely comprised of metallic elements. However, some subtleties exist within these groups:
- Group 3 (Scandium Family): Scandium, yttrium, lanthanum, and actinium are all considered metals, exhibiting typical metallic characteristics. However, their chemical properties, influenced by their complex electron configurations, aren't always straightforwardly 'metallic' in every instance.
- Group 4 (Titanium Family): Titanium, zirconium, hafnium, and rutherfordium are metals, but their high reactivity with oxygen requires special handling.
- Group 5 (Vanadium Family): Vanadium, niobium, tantalum, and dubnium are all metals but showcase a range of oxidation states, influencing their reactivity and behavior.
- Groups 6-12 (Chromium Family and others): These groups contain elements largely metallic in nature but exhibit some variances in properties. The diversity of oxidation states within these transition metals impacts their appearance, conductivity, and reactivity.
The Lanthanides and Actinides: A Special Case
The lanthanides and actinides, often placed below the main body of the periodic table, are series of inner transition metals with unique characteristics. These elements are all metals, but their properties are affected by the complex filling of their 4f and 5f orbitals, respectively. These complexities contribute to the subtle variations in their metallic properties compared to the transition metals.
The Importance of Context: Oxidation States and Allotropes
It's crucial to remember that the properties of an element can be influenced by various factors:
- Oxidation States: Many transition metals can exist in multiple oxidation states, affecting their physical and chemical properties. An element in one oxidation state might behave very differently than the same element in another. This makes classifying elements based solely on their metallic nature complex.
- Allotropes: Some elements exist in different forms (allotropes), each with unique properties. For example, carbon exists as graphite (soft, conductive) and diamond (hard, insulator). This shows how the same element, under different conditions, can display dramatically different traits.
Conclusion: No Single Group Contains Only Metals
Therefore, while groups 3-12 are predominantly composed of metals and the lanthanides and actinides are exclusively metallic, strictly defining a group containing only metals is challenging due to the nuances of metallic properties and the influences of oxidation states and allotropes. The periodic table's beauty lies in its ability to organize elements, revealing trends and relationships but also highlighting exceptions and complexities that continue to fascinate and challenge chemists to this day. The concept of "metallicity" itself is a spectrum, and not a clear-cut binary classification. Focusing on the predominant properties of elements within a group provides a more accurate understanding of their behavior. While certain groups predominantly contain metals, defining a group containing only metals is an oversimplification of the rich complexity of the periodic table.
Latest Posts
Latest Posts
-
An Electron That Has A Velocity With X Component
Mar 29, 2025
-
A Food Chain Always Starts With
Mar 29, 2025
-
How Many Players Are In A Water Polo Team
Mar 29, 2025
-
In The Figure Five Long Parallel Wires
Mar 29, 2025
-
Diagram Of A Simple Reflex Arc
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Which Periodic Table Group Contains Only Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
