Which One Is Noble Gas Metallloids
News Leon
Mar 27, 2025 · 5 min read
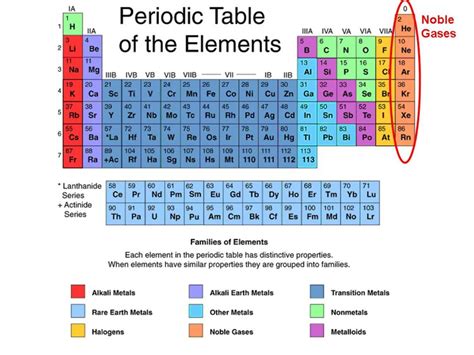
Table of Contents
Are There Noble Gas Metalloids? Unpacking the Periodic Table's Quirks
The periodic table, a seemingly simple chart, holds a wealth of information about the elements that make up our universe. Understanding the classifications within – metals, nonmetals, and metalloids – is crucial to predicting their behavior and properties. But what happens when we consider the unique group of noble gases? Can a noble gas also be a metalloid? The answer, as we'll explore in detail, is nuanced and requires a deep dive into the definition of both noble gases and metalloids.
Understanding Noble Gases: The Unreactive Elite
Noble gases, also known as inert gases, occupy Group 18 of the periodic table. They are renowned for their exceptional stability and extremely low reactivity. This unreactive nature stems from their complete valence electron shells. With their outermost electron shell fully occupied, they have little tendency to gain, lose, or share electrons, which is the basis of chemical bonding.
The noble gases include:
- Helium (He): The lightest noble gas, commonly used in balloons and cryogenics.
- Neon (Ne): Known for its bright red-orange glow in neon signs.
- Argon (Ar): The most abundant noble gas in the Earth's atmosphere, often used in welding and industrial processes.
- Krypton (Kr): Used in some lighting applications.
- Xenon (Xe): Used in specialized lighting and medical imaging.
- Radon (Rn): A radioactive gas, posing health risks due to its radioactivity.
- Oganesson (Og): A synthetic, highly radioactive element; its properties are still under investigation.
Their low reactivity makes them seemingly incompatible with the characteristics of metalloids.
Metalloids: The In-Betweeners
Metalloids, also known as semimetals, are elements that exhibit properties intermediate between metals and nonmetals. They occupy a diagonal band on the periodic table, separating the metals from the nonmetals. This intermediate nature leads to a diverse range of properties and applications. Their characteristics often depend on factors such as temperature and pressure, leading to unique behaviors.
Key characteristics of metalloids include:
- Variable Conductivity: Metalloids display electrical conductivity that's somewhere between that of metals (good conductors) and nonmetals (poor conductors). This conductivity often increases with increasing temperature, a behavior opposite to that of metals.
- Semiconductor Properties: Many metalloids are semiconductors, meaning their electrical conductivity can be controlled and manipulated. This property is crucial for the electronics industry.
- Brittle Nature: They are generally brittle solids.
- Metallic Luster (Sometimes): Some metalloids exhibit a metallic luster, though this is not always the case.
Common examples of metalloids include:
- Boron (B): Used in glass and ceramics.
- Silicon (Si): The cornerstone of the semiconductor industry.
- Germanium (Ge): Used in transistors and fiber optics.
- Arsenic (As): Used in alloys and pesticides (though its toxicity is a major concern).
- Antimony (Sb): Used in flame retardants and alloys.
- Tellurium (Te): Used in solar cells and alloys.
- Polonium (Po): A radioactive element; its extreme radioactivity limits its practical applications.
The Conflict: Can a Noble Gas Be a Metalloid?
Given the stark differences between the properties of noble gases and metalloids, it's clear to see why the idea of a noble gas metalloid seems inherently contradictory.
Noble gases are defined by their extreme reluctance to participate in chemical reactions, a direct contrast to the often complex chemical behavior seen in metalloids. Metalloids readily form compounds, showcasing a range of oxidation states, while noble gases are notoriously difficult to force into chemical reactions. Their electron configurations are fundamentally different: metalloids have incomplete valence shells, driving their reactivity, whereas noble gases possess complete shells, leading to their stability.
There are no known noble gas metalloids. The properties that define each group are mutually exclusive. While some rare compounds of heavier noble gases (like xenon) have been synthesized under extreme conditions, these reactions are exceptions that prove the rule of noble gas unreactivity. These compounds don't exhibit the typical characteristics of metalloids.
Exploring the Notion of "Metalloid-Like" Behavior (A Hypothetical Scenario)
While the idea of a noble gas metalloid is scientifically unsound, we can entertain a hypothetical scenario. Imagine a hypothetical element, possessing a highly unusual electron configuration. If this element were to exist, perhaps under extreme pressures and temperatures within a stellar environment, it might exhibit some properties that resemble those of metalloids, while also retaining some characteristics of a noble gas. However, even in this far-fetched scenario, the element would likely not meet the comprehensive definition of a metalloid. Its properties would simply represent a peculiar exception to the established rules of the periodic table.
The unique stability of noble gases stems from their electron configuration, a fundamental characteristic that resists any deviation towards the reactivity required for metalloid behavior.
The Importance of Clear Definitions in Science
The apparent conflict between the possibility of a noble gas metalloid highlights the importance of precise definitions in science. Understanding the fundamental properties that define each group of elements – metals, nonmetals, metalloids, and noble gases – allows for accurate prediction of their behaviors and potential applications. The rigorous application of these definitions prevents ambiguity and ensures consistent understanding across scientific disciplines.
Conclusion: A Clear Distinction
In conclusion, the notion of a noble gas metalloid is scientifically incompatible. The fundamental properties that define noble gases—their extreme unreactivity due to complete valence shells—are fundamentally different from the properties that characterize metalloids—their intermediate conductivity and reactivity due to incomplete valence shells. While some rare exceptions regarding reactivity of heavier noble gases exist under extreme conditions, these reactions do not lead to metalloid behavior. A clear understanding of the defining characteristics of each element group is crucial for a coherent and accurate understanding of chemistry and material science. The periodic table, while a useful tool, should be interpreted within the context of the rigorous scientific definitions that categorize elements within it.
Latest Posts
Latest Posts
-
Which Issue Does Terrace Farming Help Solve
Mar 30, 2025
-
Which Of The Following Sequences Are Geometric
Mar 30, 2025
-
A Race With Three Different Events
Mar 30, 2025
-
Is Methane A Compound Or An Element
Mar 30, 2025
-
In Uniform Circular Motion Which Of The Following Is Constant
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Which One Is Noble Gas Metallloids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
