Which Of The Following Molecules Is Polar
News Leon
Mar 18, 2025 · 5 min read
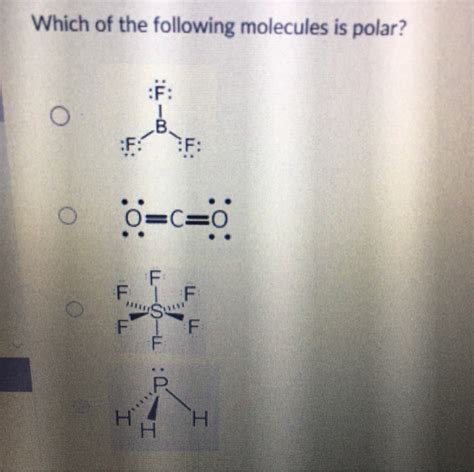
Table of Contents
Which of the Following Molecules is Polar? A Deep Dive into Molecular Polarity
Determining whether a molecule is polar or nonpolar is a fundamental concept in chemistry, crucial for understanding its properties and behavior. This article will delve deep into the factors influencing molecular polarity, providing a comprehensive guide to identifying polar molecules. We'll explore various examples and techniques, ensuring you can confidently answer the question: "Which of the following molecules is polar?"
Understanding Polarity: The Basics
Molecular polarity arises from the unequal distribution of electron density within a molecule. This unequal distribution is primarily caused by differences in electronegativity between the atoms involved. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond.
The key players:
- Electronegativity: The higher the electronegativity difference between two bonded atoms, the more polar the bond.
- Bond polarity: A polar bond is a covalent bond where electrons are shared unequally, creating a dipole moment (a separation of positive and negative charges).
- Molecular geometry: Even if a molecule contains polar bonds, the overall molecule can be nonpolar if the geometry cancels out the individual bond dipoles.
Identifying Polar Molecules: A Step-by-Step Approach
To determine if a molecule is polar, follow these steps:
-
Draw the Lewis structure: This will show you the arrangement of atoms and bonds within the molecule. This is critical for visualizing the geometry.
-
Determine the bond polarity: Compare the electronegativity values of the atoms involved in each bond. Use a periodic table or electronegativity chart as a reference. A significant difference (generally above 0.4 on the Pauling scale) indicates a polar bond.
-
Analyze the molecular geometry: Use VSEPR (Valence Shell Electron Pair Repulsion) theory to predict the molecule's three-dimensional shape. This shape is crucial because it dictates how the individual bond dipoles interact.
-
Determine the overall molecular polarity: If the individual bond dipoles cancel each other out due to symmetry, the molecule is nonpolar. If the bond dipoles do not cancel, the molecule is polar.
Examples: Polar vs. Nonpolar Molecules
Let's examine some examples to solidify our understanding.
1. Carbon Dioxide (CO₂):
- Lewis Structure: O=C=O
- Bond Polarity: The C=O bonds are polar because oxygen is significantly more electronegative than carbon.
- Molecular Geometry: Linear. The two C=O bond dipoles are equal in magnitude and point in opposite directions, resulting in a net dipole moment of zero.
- Polarity: Nonpolar
2. Water (H₂O):
- Lewis Structure: H-O-H
- Bond Polarity: The O-H bonds are polar due to the high electronegativity of oxygen.
- Molecular Geometry: Bent. The two O-H bond dipoles do not cancel each other out, resulting in a net dipole moment.
- Polarity: Polar
3. Methane (CH₄):
- Lewis Structure: A central carbon atom bonded to four hydrogen atoms.
- Bond Polarity: The C-H bonds are slightly polar, but the electronegativity difference is small.
- Molecular Geometry: Tetrahedral. The four C-H bond dipoles cancel each other out perfectly due to the symmetrical arrangement.
- Polarity: Nonpolar
4. Ammonia (NH₃):
- Lewis Structure: A central nitrogen atom bonded to three hydrogen atoms and possessing a lone pair of electrons.
- Bond Polarity: The N-H bonds are polar because nitrogen is more electronegative than hydrogen.
- Molecular Geometry: Trigonal pyramidal. The three N-H bond dipoles and the lone pair create a net dipole moment.
- Polarity: Polar
5. Carbon Tetrachloride (CCl₄):
- Lewis Structure: A central carbon atom bonded to four chlorine atoms.
- Bond Polarity: The C-Cl bonds are polar because chlorine is more electronegative than carbon.
- Molecular Geometry: Tetrahedral. The four C-Cl bond dipoles cancel each other out perfectly due to symmetry.
- Polarity: Nonpolar
Advanced Considerations: Factors Influencing Molecular Polarity
While electronegativity differences and molecular geometry are the primary determinants of polarity, some subtleties can influence the overall picture:
-
Resonance: In molecules exhibiting resonance, the delocalization of electrons can affect the overall charge distribution, potentially influencing polarity.
-
Inductive Effects: Electron-withdrawing or electron-donating groups can alter the electron density around a molecule, influencing the polarity of nearby bonds.
-
Steric Effects: Bulky substituents can sometimes influence the molecular geometry, indirectly affecting the cancellation of bond dipoles.
-
Hydrogen Bonding: This specific type of intermolecular force, significantly influencing the properties of polar molecules containing hydrogen bonded to highly electronegative atoms like oxygen, nitrogen, or fluorine, should not be confused with molecular polarity itself, although it is directly related to it.
Predicting Polarity: A Practical Approach
When faced with a question about which molecule is polar, systematically apply the steps outlined above:
-
Identify the atoms and their electronegativities: Use a periodic table or electronegativity chart.
-
Draw the Lewis structure: Visualizing the structure is essential for understanding the geometry.
-
Determine the bond polarities: Identify any significant electronegativity differences between bonded atoms.
-
Determine the molecular geometry: Utilize VSEPR theory to predict the three-dimensional shape.
-
Assess the cancellation of bond dipoles: If the dipoles cancel, the molecule is nonpolar; otherwise, it is polar.
Conclusion: Mastering Molecular Polarity
Understanding molecular polarity is critical for grasping many chemical phenomena, impacting physical properties like boiling point, melting point, solubility, and reactivity. By following a systematic approach and considering the factors discussed in this article, you'll be equipped to confidently determine which of any given set of molecules is polar. Remember to consistently apply the principles of electronegativity, molecular geometry, and bond dipole analysis for accurate predictions. This comprehensive guide provides the foundation for confidently navigating the world of molecular polarity.
Latest Posts
Latest Posts
-
What Is 25 Percent Of 25
Mar 19, 2025
-
How Do You Make A Magnet At Home
Mar 19, 2025
-
A Decrease In Demand And An Increase In Supply Will
Mar 19, 2025
-
Prove The Square Root Of 5 Is Irrational
Mar 19, 2025
-
The Figure Shown Is A Rectangle With A Semicircle
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Molecules Is Polar . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
