Which Of The Following Is An Aromatic Hydrocarbon
News Leon
Mar 30, 2025 · 5 min read
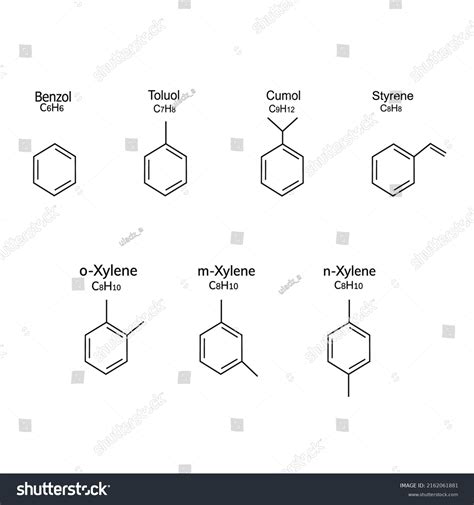
Table of Contents
Which of the Following is an Aromatic Hydrocarbon? Understanding Aromaticity
Aromatic hydrocarbons, often simply called aromatics, are a fascinating class of organic compounds with unique properties that set them apart from other hydrocarbons. Understanding what makes a molecule aromatic is crucial in organic chemistry, impacting its reactivity, stability, and applications. This comprehensive guide will delve into the definition of aromatic hydrocarbons, explore the criteria for aromaticity, and examine various examples to solidify your understanding. We'll also tackle common misconceptions and explore the significant role aromatics play in various industries.
Defining Aromatic Hydrocarbons
The term "aromatic" initially referred to compounds with pleasant odors, but the modern definition is far more precise and based on electronic structure. Aromatic hydrocarbons are cyclic, planar, conjugated molecules that follow Hückel's rule. Let's break down these crucial components:
1. Cyclic Structure:
The molecule must be a ring structure. This means the atoms are connected to form a closed loop. Linear or branched hydrocarbons are not aromatic.
2. Planar Structure:
The atoms in the ring must lie in the same plane. This allows for efficient overlap of p-orbitals, crucial for delocalized π-electron clouds. Any significant deviation from planarity disrupts aromaticity.
3. Conjugated System:
The molecule must possess a conjugated system, meaning alternating single and double bonds (or lone pairs) within the ring. This conjugation allows for the delocalization of π electrons.
4. Hückel's Rule:
This is arguably the most important criterion. Hückel's rule states that a planar, cyclic, conjugated molecule is aromatic only if it has (4n + 2) π electrons, where n is a non-negative integer (0, 1, 2, 3, and so on). This number of π electrons allows for a particularly stable electron configuration. Molecules with 4n π electrons are anti-aromatic, meaning they are highly unstable.
Examples of Aromatic Hydrocarbons
Let's analyze some examples to better grasp these criteria:
1. Benzene (C₆H₆):
Benzene is the quintessential aromatic hydrocarbon. It's a six-membered ring with alternating single and double bonds, fulfilling all the requirements for aromaticity:
- Cyclic: Yes
- Planar: Yes
- Conjugated: Yes
- π electrons: 6 (4n + 2 where n = 1)
Its exceptional stability due to the delocalized π electron cloud is a hallmark of aromatic compounds.
2. Naphthalene (C₁₀H₈):
Naphthalene consists of two fused benzene rings. It's a larger aromatic system with 10 π electrons (4n + 2 where n = 2), maintaining aromaticity in both rings.
3. Pyridine (C₅H₅N):
Pyridine is a six-membered heterocyclic aromatic compound. One carbon atom in the benzene ring is replaced by a nitrogen atom. The nitrogen atom contributes one electron to the π system, resulting in a total of 6 π electrons (4n + 2 where n = 1), maintaining aromaticity. The lone pair on the nitrogen is not part of the delocalized π system.
4. Furan (C₄H₄O):
Furan is a five-membered heterocyclic aromatic compound containing an oxygen atom. The oxygen atom contributes two electrons to the π system (one lone pair). Together with the two double bonds contributing two electrons each, the total is 6 π electrons (4n + 2 where n = 1), confirming its aromaticity. One lone pair of the oxygen remains localized and does not participate in the delocalized π system.
5. Thiophene (C₄H₄S):
Similar to furan, thiophene is a five-membered heterocyclic aromatic compound. The sulfur atom contributes two electrons from one of its lone pairs to the π system, resulting in 6 π electrons (4n + 2 where n = 1) and thus aromatic character.
Examples of Non-Aromatic Hydrocarbons
It's equally important to understand examples that do not meet the criteria for aromaticity:
1. Cyclohexane (C₆H₁₂):
Cyclohexane is a six-membered ring, but it lacks a conjugated system and has only sigma bonds. It is a saturated hydrocarbon and thus not aromatic.
2. Cyclobutadiene (C₄H₄):
Cyclobutadiene has a cyclic, conjugated structure, but it has only 4 π electrons (4n where n = 1), making it anti-aromatic and exceptionally unstable. Its square planar structure is also a factor in its instability.
3. Cyclooctatetraene (C₈H₈):
Cyclooctatetraene has 8 π electrons (4n where n = 2), which would suggest anti-aromaticity. However, to avoid the instability of an anti-aromatic system, it adopts a non-planar tub-shaped structure, which prevents the proper conjugation needed for aromaticity. It is therefore non-aromatic.
Applications of Aromatic Hydrocarbons
Aromatic hydrocarbons play a pivotal role in various industries:
- Petrochemicals: Benzene, toluene, and xylenes (BTX) are crucial building blocks for the production of plastics, synthetic fibers, and other chemicals.
- Pharmaceuticals: Many pharmaceuticals contain aromatic rings as part of their molecular structure. Understanding their reactivity is crucial for drug design and synthesis.
- Polymers: Aromatic polymers like polyesters and polycarbonates are used in various applications, from clothing to construction materials.
- Dyes and Pigments: Many dyes and pigments contain aromatic structures, contributing to their vibrant colors and stability.
- Solvents: Aromatic hydrocarbons, such as toluene and benzene (though its use is now restricted due to its toxicity), were historically used as solvents in various industrial processes.
Safety Considerations
It's crucial to acknowledge the potential health hazards associated with certain aromatic hydrocarbons. Benzene, in particular, is known to be carcinogenic. Proper safety precautions and handling procedures are necessary when working with these compounds. Always consult the relevant Safety Data Sheets (SDS) before handling any chemical.
Conclusion: Identifying Aromatic Hydrocarbons
Determining whether a compound is aromatic requires a careful examination of its structure. The cyclic, planar, conjugated nature of the molecule, combined with adherence to Hückel's rule (4n + 2 π electrons), are the key determinants. Understanding aromaticity is essential not only for organic chemistry but also for various fields that rely on the synthesis and application of aromatic hydrocarbons. This knowledge allows us to predict reactivity, understand stability, and develop innovative applications in diverse sectors. Remember that while many aromatics offer significant benefits, it’s essential to handle them safely, always prioritizing your health and the environment.
Latest Posts
Latest Posts
-
Weak Acid And Weak Base Ph
Apr 01, 2025
-
What Is The Measure Of Angle B In Degrees
Apr 01, 2025
-
What Is The Formula Of Iq
Apr 01, 2025
-
A Path That An Electric Current Follows Is A
Apr 01, 2025
-
Distance From Earth To Sun Scientific Notation
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is An Aromatic Hydrocarbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
