Which Of The Following Is A Tertiary Alcohol
News Leon
Mar 15, 2025 · 6 min read
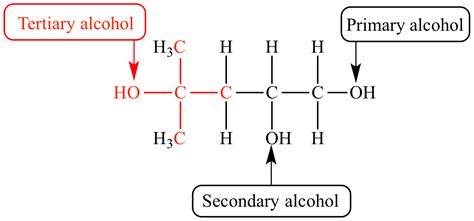
Table of Contents
Which of the Following is a Tertiary Alcohol? A Comprehensive Guide
Understanding the classification of alcohols is fundamental in organic chemistry. Alcohols are organic compounds containing a hydroxyl (-OH) group bonded to a carbon atom. This seemingly simple structure leads to a diverse range of properties and reactivity, largely influenced by the number of carbon atoms bonded to the carbon atom bearing the hydroxyl group. This branching determines whether an alcohol is classified as primary (1°), secondary (2°), or tertiary (3°). This article delves deep into the identification of tertiary alcohols, explaining their structure, properties, and differentiating them from primary and secondary alcohols.
Understanding Alcohol Classification: Primary, Secondary, and Tertiary
The classification of alcohols hinges on the number of alkyl groups (carbon chains) attached to the carbon atom directly bonded to the hydroxyl group (-OH).
Primary Alcohols (1°)
A primary alcohol has the hydroxyl group attached to a primary carbon atom. A primary carbon atom is bonded to only one other carbon atom.
Example: Methanol (CH₃OH) and Ethanol (CH₃CH₂OH) are primary alcohols.
Secondary Alcohols (2°)
A secondary alcohol has the hydroxyl group attached to a secondary carbon atom. A secondary carbon atom is bonded to two other carbon atoms.
Example: Propan-2-ol (CH₃CH(OH)CH₃) is a secondary alcohol.
Tertiary Alcohols (3°)
A tertiary alcohol has the hydroxyl group attached to a tertiary carbon atom. A tertiary carbon atom is bonded to three other carbon atoms.
Example: 2-Methylpropan-2-ol ((CH₃)₃COH) is a tertiary alcohol. This is because the carbon atom connected to the -OH group is bonded to three methyl groups (CH₃).
Identifying Tertiary Alcohols: A Step-by-Step Approach
Identifying a tertiary alcohol requires careful examination of its structural formula. Here’s a systematic approach:
-
Locate the Hydroxyl Group (-OH): First, identify the hydroxyl group within the molecule. This is crucial as it's the defining feature of an alcohol.
-
Identify the Carbon Atom Bonded to the -OH Group: Pinpoint the carbon atom directly connected to the hydroxyl group. This carbon atom is the key to classification.
-
Count the Carbon-Carbon Bonds: Count the number of carbon-carbon single bonds connected to the carbon atom bearing the hydroxyl group.
-
Classification:
- One carbon-carbon bond: Primary alcohol (1°)
- Two carbon-carbon bonds: Secondary alcohol (2°)
- Three carbon-carbon bonds: Tertiary alcohol (3°)
Examples of Tertiary Alcohols and Their Structures
Let's examine several examples of tertiary alcohols to solidify understanding:
-
2-Methylpropan-2-ol ((CH₃)₃COH): The carbon atom linked to the -OH is bonded to three methyl groups (CH₃). This is a classic example of a tertiary alcohol.
-
2,3-Dimethylbutan-2-ol: This molecule possesses a tertiary carbon atom connected to the hydroxyl group. It is linked to three other carbon atoms. The presence of the methyl groups doesn't alter the classification.
-
tert-Butyl alcohol: This is another name for 2-Methylpropan-2-ol, highlighting the tertiary butyl group attached to the -OH.
-
3,3-Dimethyl-2-butanol: The carbon atom carrying the hydroxyl group is attached to three carbon atoms, making it a tertiary alcohol.
-
1,1-Dimethylcyclohexanol: Although part of a cyclic structure, the carbon bearing the –OH group is linked to three other carbons. This qualifies it as a tertiary alcohol.
Differentiating Tertiary Alcohols from Primary and Secondary Alcohols
The key distinction lies in the degree of substitution of the carbon atom connected to the hydroxyl group. A simple comparison table highlights the differences:
| Feature | Primary Alcohol (1°) | Secondary Alcohol (2°) | Tertiary Alcohol (3°) |
|---|---|---|---|
| Carbon attached to -OH | Bonded to 1 carbon | Bonded to 2 carbons | Bonded to 3 carbons |
| Number of alkyl groups | 1 | 2 | 3 |
| Reactivity | Most reactive | Less reactive than 1° | Least reactive |
| Oxidation Products | Aldehyde then carboxylic acid | Ketone | Resistant to oxidation |
Chemical Properties and Reactivity of Tertiary Alcohols
Tertiary alcohols exhibit distinct chemical properties compared to primary and secondary alcohols. This difference in reactivity stems from the steric hindrance caused by the three alkyl groups surrounding the hydroxyl group. This steric bulk inhibits the approach of reactants, affecting the rate of many reactions.
Oxidation
One significant difference is their resistance to oxidation. Unlike primary and secondary alcohols, tertiary alcohols do not undergo oxidation under normal conditions. This is because oxidation involves the breaking of a C-H bond adjacent to the hydroxyl group, and tertiary alcohols lack such a bond. Primary alcohols oxidize to aldehydes and then carboxylic acids, while secondary alcohols oxidize to ketones. The absence of a C-H bond alpha to the hydroxyl group prevents oxidation in tertiary alcohols.
Dehydration
Tertiary alcohols undergo dehydration (removal of water) more readily than primary or secondary alcohols. The mechanism involves the formation of a carbocation intermediate. The stability of the carbocation, directly related to the number of alkyl groups, influences the reaction rate. Tertiary carbocations are the most stable, leading to faster dehydration reactions.
Esterification
Tertiary alcohols react with carboxylic acids to form esters, but the reaction is significantly slower than for primary and secondary alcohols due to steric hindrance.
Applications of Tertiary Alcohols
Tertiary alcohols find various applications across different industries:
-
Solvents: Their solubility in both polar and non-polar solvents makes them valuable solvents in various chemical processes.
-
Intermediates in Organic Synthesis: They serve as crucial intermediates in the synthesis of various organic compounds. Their dehydration to alkenes is a vital step in several synthetic pathways.
-
Pharmaceuticals: Some tertiary alcohols possess significant biological activity and are incorporated into the structure of certain pharmaceuticals.
-
Fragrances and Flavors: Certain tertiary alcohols contribute to the characteristic odor and taste of some perfumes and flavorings.
Solving Problems: Identifying Tertiary Alcohols in a Set of Compounds
Let's tackle a few examples to solidify our understanding. Identify the tertiary alcohol in each set:
Set 1:
- Ethanol (CH₃CH₂OH): Primary alcohol
- Propan-2-ol (CH₃CH(OH)CH₃): Secondary alcohol
- 2-Methylpropan-2-ol ((CH₃)₃COH): Tertiary alcohol
Set 2:
- Butan-1-ol: Primary alcohol
- 2-Methylpropan-1-ol: Primary alcohol
- 2,2-Dimethylpropan-1-ol: Primary alcohol
- 2,2-Dimethylpropan-2-ol: Tertiary alcohol
Set 3:
- Cyclohexanol: Secondary alcohol
- 3-methylcyclohexanol: Secondary alcohol
- 2-methylcyclohexanol: Secondary alcohol
- tert-butylcyclohexanol: Tertiary alcohol (The -OH is attached to a carbon bonded to three other carbons, including the cyclohexyl ring)
Conclusion
Understanding the classification of alcohols, particularly distinguishing tertiary alcohols, is crucial for anyone studying organic chemistry. The structural differences profoundly impact their chemical properties and reactivity. By systematically analyzing the carbon atoms bonded to the hydroxyl group, we can confidently identify tertiary alcohols and predict their behaviour in various chemical reactions. Remember that the key is recognizing the tertiary carbon atom – a carbon atom bonded to three other carbon atoms – directly attached to the hydroxyl group. This simple rule allows accurate and efficient identification of these important organic compounds. The unique properties of tertiary alcohols, particularly their resistance to oxidation and their relatively facile dehydration, open up a wide range of applications in diverse fields. Mastering the identification and understanding the properties of tertiary alcohols is an essential step in advancing your knowledge of organic chemistry.
Latest Posts
Latest Posts
-
Iodine Is Essential For The Synthesis Of
Mar 15, 2025
-
Is Osmosis High To Low Or Low To High
Mar 15, 2025
-
Concave Mirror And Convex Mirror Difference
Mar 15, 2025
-
Which Is Not A Cranial Bone Of The Skull
Mar 15, 2025
-
Mountain Range That Separates Europe And Asia
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Tertiary Alcohol . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
