Is Osmosis High To Low Or Low To High
News Leon
Mar 15, 2025 · 6 min read
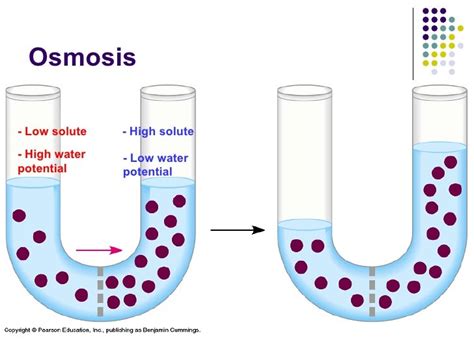
Table of Contents
Is Osmosis High to Low or Low to High? Understanding Water Movement Across Membranes
Osmosis, a fundamental process in biology and chemistry, governs the movement of water across selectively permeable membranes. Understanding the direction of this movement is crucial for grasping many biological phenomena, from the function of cell membranes to the transport of nutrients in plants. The simple answer is that osmosis is always high to low, referring to the concentration of water, not the concentration of solute. However, unpacking this seemingly straightforward statement requires a deeper understanding of water potential, solute concentration, and the driving force behind osmosis.
Understanding Water Potential: The Driving Force of Osmosis
Instead of simply thinking about the concentration of solute, we should think about water potential. Water potential (Ψ) is the measure of the free energy of water. It represents the tendency of water to move from one area to another. Water potential is affected by two major components:
-
Solute potential (ΨS): This component reflects the effect of dissolved solutes on the water potential. The presence of solutes lowers the water potential because the solutes bind water molecules, reducing their availability to move. A higher solute concentration means a more negative solute potential. Pure water has a solute potential of zero.
-
Pressure potential (ΨP): This component accounts for the physical pressure on the water. Positive pressure potential (turgor pressure in plant cells) increases water potential, while negative pressure potential (tension in the xylem of plants) decreases it.
The total water potential is the sum of these two components: Ψ = ΨS + ΨP. Water always moves from an area of higher water potential to an area of lower water potential. This movement continues until equilibrium is reached, where the water potential is equal on both sides of the membrane.
Osmosis: A Closer Look at Water Movement
The selectively permeable membrane plays a crucial role in osmosis. It allows water molecules to pass through but restricts the movement of larger solute molecules. This differential permeability creates the conditions for water movement driven by differences in water potential.
Imagine two solutions separated by a selectively permeable membrane: solution A with a high water concentration (low solute concentration) and solution B with a low water concentration (high solute concentration). Solution A has a higher water potential (less negative) than solution B. Therefore, water moves from solution A (higher water potential) to solution B (lower water potential) across the membrane by osmosis. This continues until the water potential on both sides becomes equal, or until a counteracting pressure (pressure potential) prevents further movement.
Why the "High to Low" Terminology Can Be Misleading
The common phrase "osmosis is high to low" can be somewhat misleading if not carefully explained. It is crucial to specify that this refers to the concentration of water, not the concentration of solutes. A solution with a high concentration of solute actually has a low concentration of water and therefore a low water potential. Conversely, a solution with a low concentration of solute has a high concentration of water and a high water potential.
Therefore, the movement is always from a region of high water concentration (high water potential) to a region of low water concentration (low water potential). Focusing solely on solute concentration without considering water potential can lead to confusion.
Osmosis in Biological Systems: Examples and Applications
Osmosis plays a vital role in numerous biological processes. Understanding these examples further clarifies the directionality of water movement:
1. Plant Cells: Turgor Pressure and Wilting
Plant cells maintain their turgor pressure through osmosis. When a plant cell is placed in hypotonic solution (a solution with a lower solute concentration than the cell's cytoplasm), water moves into the cell, increasing its turgor pressure. This pressure pushes the cell membrane against the cell wall, maintaining the cell's shape and rigidity. Conversely, in a hypertonic solution (higher solute concentration), water moves out of the cell, causing the cell to plasmolyze (shrink away from the cell wall), leading to wilting.
2. Animal Cells: Maintaining Cell Volume and Function
Animal cells do not have cell walls, making them more susceptible to changes in osmotic pressure. In a hypotonic solution, animal cells can undergo lysis (bursting) due to the influx of water. In a hypertonic solution, animal cells crenate (shrink) as water moves out. Maintaining the appropriate osmotic balance is crucial for the proper functioning of animal cells.
3. Water Absorption in Plants: Root Hairs and Xylem
Water absorption by plant roots relies heavily on osmosis. Root hairs have a high water potential compared to the soil solution, driving water into the root cells. This water then moves through the root cortex to the xylem vessels, where it is transported to other parts of the plant.
4. Kidney Function: Maintaining Blood Osmolarity
The kidneys play a vital role in regulating blood osmolarity (the concentration of solutes in the blood) through osmosis. They selectively reabsorb water and solutes to maintain the proper balance of water and electrolytes in the body. This process is crucial for maintaining blood pressure and overall homeostasis.
5. Cell Membrane Function: Selective Permeability and Transport
The cell membrane’s selective permeability is key to regulating the passage of water and other substances. While osmosis focuses on water, other transport mechanisms like facilitated diffusion and active transport manage the movement of other molecules. Understanding osmosis lays the foundation for understanding these other crucial cellular processes.
Practical Applications and Beyond Biology
The principles of osmosis extend beyond biological systems, with significant implications in various fields:
-
Desalination: Osmosis is a key principle in desalination technologies, which remove salt from seawater to produce potable water. Reverse osmosis forces water through a semi-permeable membrane against the osmotic gradient, separating the salt from the water.
-
Food preservation: Osmosis plays a role in food preservation techniques like pickling and jam making. The high solute concentration in brine or sugar solutions draws water out of microorganisms, inhibiting their growth and preserving the food.
-
Medical applications: Osmosis is used in dialysis, a treatment for kidney failure, where waste products and excess water are removed from the blood through a semi-permeable membrane.
Addressing Common Misconceptions
Several misunderstandings surrounding osmosis often arise:
-
Osmosis is only about water: While osmosis primarily concerns water movement, it's critical to remember that the movement is driven by water potential, influenced by both solute and pressure potentials.
-
Osmosis only moves from low concentration to high concentration: This is incorrect. Osmosis moves from high water potential to low water potential, which can correlate with low solute concentration to high solute concentration but isn’t solely determined by the solute concentration itself.
-
Osmosis is always fast: The rate of osmosis depends on several factors, including the concentration gradient, the permeability of the membrane, and the temperature.
Conclusion: Mastering the Nuances of Osmosis
Understanding osmosis requires grasping the concept of water potential and its components. While the simplified phrase "high to low" is a useful mnemonic, it's crucial to remember that this refers to water potential, not solely solute concentration. By recognizing the interplay between solute potential and pressure potential, and appreciating the role of selectively permeable membranes, one can fully grasp the complexities and biological significance of this fundamental process. Osmosis is not merely a passive movement of water; it's an active driver of vital biological functions and has crucial implications in numerous scientific and technological applications.
Latest Posts
Latest Posts
-
Is Boiling Water A Physical Change
Mar 17, 2025
-
Is A Webcam An Input Or Output Device
Mar 17, 2025
-
Word For A Person Who Uses Big Words
Mar 17, 2025
-
What Is 375 As A Percentage
Mar 17, 2025
-
Which Is The Correct Order Of The Scientific Method
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Osmosis High To Low Or Low To High . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
