Which Of The Following Is A Carboxylic Acid
News Leon
Mar 23, 2025 · 6 min read
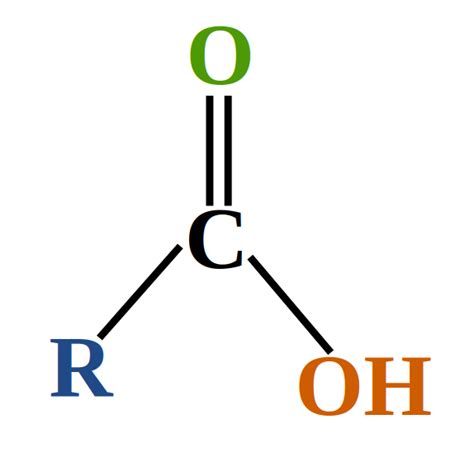
Table of Contents
Which of the Following is a Carboxylic Acid? A Deep Dive into Organic Chemistry
Identifying carboxylic acids among various organic compounds is a fundamental skill in organic chemistry. This comprehensive guide will not only answer the question "Which of the following is a carboxylic acid?" but will also equip you with the knowledge to confidently identify them in any context. We'll explore the defining characteristics of carboxylic acids, delve into their nomenclature, examine their properties, and illustrate their importance in various applications.
Understanding Carboxylic Acids: The Defining Functional Group
Carboxylic acids are a class of organic compounds characterized by the presence of a carboxyl group. This functional group consists of a carbonyl group (C=O) bonded to a hydroxyl group (-OH). The general formula for a carboxylic acid is R-COOH, where 'R' represents an alkyl or aryl group (a hydrocarbon chain or ring). The presence of this specific carboxyl group (-COOH) is the key identifier.
Key Characteristics of the Carboxyl Group
- Polarity: The carboxyl group is highly polar due to the presence of both the carbonyl and hydroxyl groups. This polarity significantly influences the physical and chemical properties of carboxylic acids.
- Acidity: The hydroxyl group's hydrogen atom is readily ionizable, leading to the acidic nature of carboxylic acids. This is due to resonance stabilization of the carboxylate ion (R-COO⁻) formed after proton donation. The acidic nature is crucial for many of their reactions and applications.
- Hydrogen Bonding: The presence of both the carbonyl and hydroxyl groups allows for extensive hydrogen bonding between carboxylic acid molecules. This intermolecular interaction results in higher boiling points compared to other organic compounds of similar molecular weight.
Identifying Carboxylic Acids: A Practical Approach
Let's look at a systematic approach to identifying carboxylic acids in a given list of organic compounds. This method will help you confidently differentiate carboxylic acids from other functional groups like alcohols, ketones, aldehydes, and esters.
Step-by-Step Identification Process
- Look for the -COOH group: The most straightforward way to identify a carboxylic acid is to directly look for the presence of the carboxyl group (-COOH) in the structural formula of the compound. This group is the defining characteristic.
- Recognize the carbonyl and hydroxyl groups: Identify the presence of both a carbonyl group (C=O) directly attached to a hydroxyl group (-OH). These two groups, combined, constitute the carboxyl group.
- Consider the IUPAC nomenclature: Carboxylic acids typically end with the suffix "-oic acid" in their IUPAC names. For example, CH₃COOH is called ethanoic acid (acetic acid in common nomenclature). This nomenclature provides a strong clue.
- Analyze the properties: Carboxylic acids generally exhibit high boiling points due to hydrogen bonding and are acidic in nature. These properties can help confirm the presence of the functional group, although other factors may influence the exact values.
Differentiating Carboxylic Acids from Other Functional Groups
It's crucial to distinguish carboxylic acids from other functional groups with similar structural features. Understanding the subtle differences is key to accurate identification.
1. Carboxylic Acids vs. Alcohols
Both carboxylic acids and alcohols contain a hydroxyl group (-OH). However, in carboxylic acids, this hydroxyl group is directly bonded to a carbonyl carbon (C=O), forming the carboxyl group. In alcohols, the hydroxyl group is bonded to a carbon atom that is not part of a carbonyl group.
Example:
- Carboxylic acid: CH₃COOH (Acetic acid) – -OH bonded to C=O
- Alcohol: CH₃CH₂OH (Ethanol) – -OH bonded to a carbon without a C=O
2. Carboxylic Acids vs. Ketones
Ketones contain a carbonyl group (C=O) bonded to two carbon atoms. Carboxylic acids have a carbonyl group bonded to one carbon and one hydroxyl group (-OH).
Example:
- Carboxylic acid: CH₃COOH (Acetic acid) – C=O bonded to -OH and CH₃
- Ketone: CH₃COCH₃ (Acetone) – C=O bonded to two CH₃ groups
3. Carboxylic Acids vs. Aldehydes
Aldehydes, like ketones, have a carbonyl group (C=O), but the carbonyl carbon is bonded to at least one hydrogen atom. Carboxylic acids have a carbonyl carbon bonded to a hydroxyl group (-OH).
Example:
- Carboxylic acid: CH₃COOH (Acetic acid) – C=O bonded to -OH and CH₃
- Aldehyde: CH₃CHO (Ethanal) – C=O bonded to H and CH₃
4. Carboxylic Acids vs. Esters
Esters are derived from carboxylic acids through esterification. They have a similar carbonyl group but instead of a hydroxyl group (-OH), they have an alkoxy group (-OR), where 'R' is an alkyl group.
Example:
- Carboxylic acid: CH₃COOH (Acetic acid) – C=O bonded to -OH
- Ester: CH₃COOCH₃ (Methyl acetate) – C=O bonded to -OCH₃
The Importance of Carboxylic Acids: Applications and Significance
Carboxylic acids are ubiquitous in nature and play vital roles in various biological and industrial processes. Their acidic properties and ability to form various derivatives make them essential building blocks and functional components in numerous applications.
Biological Significance:
- Amino Acids: The building blocks of proteins are all α-amino acids, possessing both an amino group (-NH₂) and a carboxylic acid group (-COOH).
- Fatty Acids: Long-chain carboxylic acids are crucial components of lipids and fats, playing essential roles in cell membranes and energy storage.
- Citric Acid Cycle: This vital metabolic pathway heavily relies on several carboxylic acids, enabling energy production within cells.
- Acetic Acid: A crucial component in vinegar, acetic acid is also involved in numerous metabolic processes.
Industrial Applications:
- Polymers: Carboxylic acids serve as monomers in the synthesis of numerous polymers, such as polyesters and polyamides (nylons).
- Pharmaceuticals: Many drugs and pharmaceutical compounds contain carboxylic acid groups, contributing to their biological activity.
- Food Industry: Carboxylic acids are used as preservatives, flavoring agents, and acidity regulators in food products.
- Solvents and Cleaning Agents: Some carboxylic acids and their derivatives are used as solvents and cleaning agents due to their polarity and ability to dissolve various substances.
- Textile Industry: Carboxylic acids and their derivatives play a critical role in the production and processing of textiles.
Advanced Concepts and Further Exploration
Beyond the basics, several advanced concepts related to carboxylic acids are worth exploring for a deeper understanding of their chemistry.
1. Acid Dissociation Constants (pKa):** Understanding the pKa values of carboxylic acids allows for prediction of their acidity and behavior in different chemical environments. The lower the pKa value, the stronger the acid.
2. Esterification:** The reaction of a carboxylic acid with an alcohol to form an ester is a crucial synthetic process in organic chemistry. This reaction is typically catalyzed by an acid.
3. Decarboxylation:** The removal of a carboxyl group from a carboxylic acid is a significant transformation, often producing carbon dioxide as a byproduct. This reaction has implications in various chemical and biological processes.
4. Reduction:** Carboxylic acids can be reduced to primary alcohols using reducing agents like lithium aluminum hydride (LiAlH₄).
5. Reactions with Grignard Reagents:** Grignard reagents react with carboxylic acids to produce tertiary alcohols.
Conclusion
Identifying carboxylic acids hinges on understanding their defining carboxyl functional group (-COOH) and its unique properties. By systematically analyzing structural formulas, IUPAC nomenclature, and chemical properties, one can confidently distinguish carboxylic acids from other organic compounds. Their widespread presence and diverse applications underscore their importance in both biological systems and numerous industrial processes. This comprehensive guide provided the foundational knowledge necessary to confidently answer, "Which of the following is a carboxylic acid?" and to further explore the fascinating world of organic chemistry. Remember to practice identifying different functional groups to solidify your understanding. The more you practice, the easier it will become to recognize these essential building blocks of organic chemistry.
Latest Posts
Latest Posts
-
Desert That Covers Most Of North Africa
Mar 25, 2025
-
Draw The Organic Product S Of The Following Reaction
Mar 25, 2025
-
What Is The Density Of Aluminium
Mar 25, 2025
-
Which Statement Correctly Describes Magnetic Field Lines
Mar 25, 2025
-
Integration Of X 2e X 2
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Is A Carboxylic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
