Draw The Organic Product S Of The Following Reaction
News Leon
Mar 25, 2025 · 6 min read
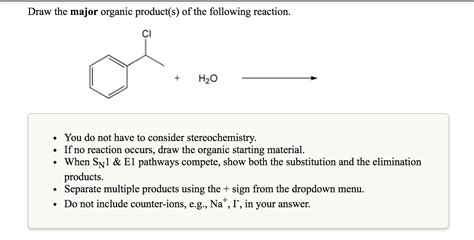
Table of Contents
Predicting Organic Product Outcomes: A Deep Dive into Reaction Mechanisms
Predicting the organic products of a chemical reaction is a cornerstone of organic chemistry. It requires a thorough understanding of reaction mechanisms, functional groups, and the principles of thermodynamics and kinetics. This article will delve into the intricacies of predicting organic product outcomes, using examples to illustrate key concepts. We’ll explore various reaction types and the factors that influence the formation of specific products. Remember, while predicting products is crucial, performing the reaction in a lab setting and analyzing the obtained products through techniques like NMR and IR spectroscopy is always necessary to confirm the predictions.
Understanding Reaction Mechanisms: The Key to Prediction
Before we dive into specific reactions, it's vital to grasp the concept of reaction mechanisms. A reaction mechanism describes the step-by-step process by which reactants are transformed into products. It details the bond breaking and bond forming events that occur at the molecular level. Understanding these mechanisms allows us to anticipate what products will form under specific conditions. Mechanisms often involve:
- Nucleophilic attack: A nucleophile (electron-rich species) attacks an electrophile (electron-deficient species).
- Electrophilic attack: An electrophile attacks a nucleophile.
- Elimination reactions: Loss of atoms or groups from a molecule, often leading to the formation of a double or triple bond.
- Addition reactions: Addition of atoms or groups to a molecule, often to a double or triple bond.
- Rearrangements: Rearrangement of atoms within a molecule.
Factors Influencing Product Formation
Several factors can influence which products are formed in a reaction:
- Substrate Structure: The structure of the starting material significantly impacts the reaction pathway and product formation. Functional groups, steric hindrance, and the presence of chiral centers all play a role.
- Reagents: The choice of reagents can dramatically alter the reaction outcome. Different reagents can favor different pathways, leading to distinct products. Consider the strength and nature of the nucleophile or electrophile employed.
- Reaction Conditions: Temperature, solvent, pressure, and the presence of catalysts can all influence the reaction pathway and the product distribution. For example, higher temperatures often favor faster reactions and might lead to different product ratios compared to lower temperatures.
- Thermodynamics and Kinetics: Thermodynamic control favors the most stable product, whereas kinetic control favors the product that is formed fastest. The relative importance of thermodynamics and kinetics depends on the reaction conditions.
Examples of Reaction Types and Product Prediction
Let's explore some common reaction types and illustrate how to predict the organic products:
1. SN1 and SN2 Reactions
These reactions are nucleophilic substitutions, where a nucleophile replaces a leaving group on a carbon atom.
-
SN2 Reaction: A concerted mechanism where the nucleophile attacks from the backside of the leaving group, leading to inversion of configuration at the chiral center (if present). This reaction is favored by strong nucleophiles, primary substrates, and polar aprotic solvents.
-
SN1 Reaction: A two-step mechanism involving the formation of a carbocation intermediate. The nucleophile then attacks the carbocation, leading to a racemic mixture if the carbocation is not chiral. This reaction is favored by weak nucleophiles, tertiary substrates, and polar protic solvents.
Example: Reaction of 2-bromobutane with sodium hydroxide (NaOH) can proceed via either SN1 or SN2, depending on the conditions. In a polar aprotic solvent with high concentration of NaOH (strong nucleophile), an SN2 reaction will dominate, resulting in 2-butanol with inversion of configuration. In a polar protic solvent with low concentration of NaOH (weak nucleophile), an SN1 reaction will be favoured, producing a racemic mixture of 2-butanol.
2. E1 and E2 Elimination Reactions
These reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, leading to the formation of a double bond (alkene).
-
E2 Reaction: A concerted mechanism where the base abstracts a proton and the leaving group departs simultaneously. This reaction often leads to the formation of the more substituted alkene (Zaitsev's rule), favored by strong bases and secondary or tertiary substrates.
-
E1 Reaction: A two-step mechanism involving the formation of a carbocation intermediate, followed by the loss of a proton to form the alkene. This reaction also often follows Zaitsev's rule but can lead to more rearrangements due to carbocation instability. Favoured by weak bases and tertiary substrates.
Example: Dehydration of 2-methyl-2-propanol using concentrated sulfuric acid as a catalyst. This is an E1 reaction leading primarily to 2-methylpropene (isobutylene), the more substituted alkene.
3. Addition Reactions
Addition reactions involve the addition of atoms or groups to a double or triple bond, breaking the pi bond.
-
Electrophilic Addition: An electrophile attacks the double bond, forming a carbocation intermediate which is then attacked by a nucleophile. Markovnikov's rule often dictates the regioselectivity, with the electrophile adding to the more substituted carbon.
-
Nucleophilic Addition: A nucleophile attacks a carbonyl group (C=O), forming a tetrahedral intermediate. This is common in reactions with aldehydes and ketones.
Example: Addition of HBr to propene. This is an electrophilic addition, following Markovnikov's rule, resulting in 2-bromopropane.
4. Oxidation and Reduction Reactions
These reactions involve the change in the oxidation state of a carbon atom. Oxidizing agents increase the oxidation state (e.g., converting alcohols to ketones or carboxylic acids), while reducing agents decrease it (e.g., converting ketones to alcohols).
Example: Oxidation of a primary alcohol using chromic acid (H2CrO4) will produce a carboxylic acid. Oxidation of a secondary alcohol will yield a ketone.
5. Grignard Reactions
Grignard reagents (RMgX) are organometallic compounds that act as strong nucleophiles. They react with carbonyl compounds (aldehydes, ketones, esters, etc.) to form new carbon-carbon bonds.
Example: Reaction of methylmagnesium bromide (CH3MgBr) with formaldehyde (HCHO) yields primary alcohol after acidic workup. Reaction with a ketone will produce a tertiary alcohol.
Advanced Concepts and Considerations
Predicting product outcomes can become considerably more complex when dealing with:
-
Stereochemistry: Understanding stereochemistry (the three-dimensional arrangement of atoms) is crucial for predicting the stereochemical outcome of reactions. Consider the possibility of diastereomers and enantiomers.
-
Regioselectivity: This refers to the preference for one constitutional isomer over another. Factors like Markovnikov's rule and Zaitsev's rule help predict regioselectivity.
-
Chemoselectivity: This refers to the preference for one functional group to react over another. Protecting groups can be used to control chemoselectivity.
Conclusion: Mastering Product Prediction
Predicting the organic products of a reaction is a complex but rewarding skill. By developing a strong understanding of reaction mechanisms, functional groups, and the various factors influencing reactivity, you can significantly improve your ability to accurately predict reaction outcomes. Remember that this is a skill built through practice and exposure to a wide range of reactions. Supplement your theoretical understanding with laboratory work and spectroscopic analysis to verify your predictions and gain a deeper understanding of organic chemistry. Always double-check your work and consider the limitations of your predictions. This iterative process of prediction, experimentation, and analysis is central to the advancement of organic chemistry itself.
Latest Posts
Latest Posts
-
Genes Had Been Absent On The Chromosomes
Mar 28, 2025
-
Which Of The Following Is A Nonrenewable Source Of Energy
Mar 28, 2025
-
An Improvement In Production Technology Will
Mar 28, 2025
-
If 2 X 1 14 Then X
Mar 28, 2025
-
How Many Mm Are In 50 Cm
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Draw The Organic Product S Of The Following Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
