Which Of The Following Has The Lowest Boiling Point
News Leon
Mar 15, 2025 · 6 min read
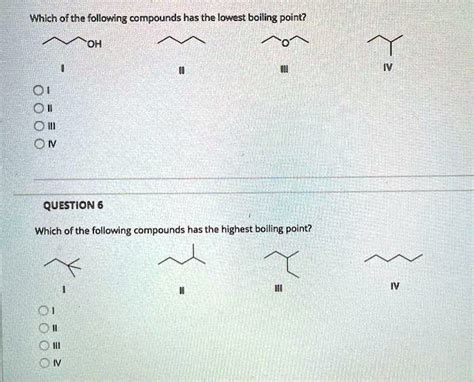
Table of Contents
Which of the Following Has the Lowest Boiling Point? Understanding Intermolecular Forces and Boiling Point
Determining which substance possesses the lowest boiling point from a given set requires understanding the fundamental principles governing intermolecular forces and their relationship to boiling points. Boiling point is the temperature at which a substance transitions from a liquid to a gas. This transition requires overcoming the attractive forces holding the molecules together in the liquid phase. The stronger these intermolecular forces, the higher the boiling point. Conversely, weaker intermolecular forces result in lower boiling points.
This article will delve into the various types of intermolecular forces, explore their relative strengths, and provide a practical framework for predicting which substance in a given group will exhibit the lowest boiling point. We will also look at specific examples to solidify your understanding.
Understanding Intermolecular Forces
Intermolecular forces are the attractive forces between molecules. These forces are significantly weaker than the intramolecular forces (bonds within a molecule), but they are crucial in determining a substance's physical properties, including its boiling point. The primary types of intermolecular forces are:
1. London Dispersion Forces (LDFs)
These are the weakest type of intermolecular force and are present in all molecules, regardless of their polarity. LDFs arise from temporary, instantaneous dipoles created by the random movement of electrons within a molecule. These temporary dipoles induce dipoles in neighboring molecules, leading to a weak attractive force. The strength of LDFs generally increases with the size and shape of the molecule. Larger molecules with more electrons have greater opportunities for temporary dipole formation, leading to stronger LDFs.
Key characteristics:
- Present in all molecules.
- Strength increases with molecular size and surface area.
- Weakest type of intermolecular force.
2. Dipole-Dipole Forces
These forces occur between polar molecules – molecules with a permanent dipole moment due to an uneven distribution of electron density. The positive end of one polar molecule attracts the negative end of another, resulting in a stronger attractive force compared to LDFs.
Key characteristics:
- Present in polar molecules only.
- Stronger than LDFs.
- Strength depends on the magnitude of the dipole moment.
3. Hydrogen Bonding
This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (oxygen, nitrogen, or fluorine). The highly electronegative atom strongly attracts the electrons in the hydrogen bond, creating a strong dipole. Hydrogen bonds are significantly stronger than typical dipole-dipole forces and are responsible for many of water's unique properties.
Key characteristics:
- Strongest type of intermolecular force.
- Occurs only when hydrogen is bonded to O, N, or F.
- Responsible for many unique properties of water and other molecules.
Predicting Boiling Point Based on Intermolecular Forces
To determine which substance has the lowest boiling point, follow these steps:
-
Identify the types of intermolecular forces present: Determine whether each molecule is polar or nonpolar. Polar molecules will exhibit dipole-dipole forces, and those with O-H, N-H, or F-H bonds will have hydrogen bonding. All molecules will have LDFs.
-
Compare the strengths of intermolecular forces: The substance with the weakest overall intermolecular forces will have the lowest boiling point. Hydrogen bonding is the strongest, followed by dipole-dipole forces, and then LDFs.
-
Consider molecular size and shape: For molecules with similar types of intermolecular forces, larger molecules with greater surface area will generally have stronger LDFs and thus higher boiling points. Branching can also affect boiling point; more branched molecules have lower boiling points than their linear isomers due to decreased surface area contact.
Examples: Comparing Boiling Points
Let's consider some examples to illustrate the concepts discussed:
Example 1: Compare the boiling points of methane (CH₄), ethanol (CH₃CH₂OH), and water (H₂O).
- Methane (CH₄): Nonpolar, only LDFs are present.
- Ethanol (CH₃CH₂OH): Polar, exhibits LDFs, dipole-dipole forces, and hydrogen bonding (due to the O-H bond).
- Water (H₂O): Polar, exhibits LDFs, dipole-dipole forces, and strong hydrogen bonding.
Conclusion: Methane will have the lowest boiling point because it only has weak LDFs. Ethanol will have a higher boiling point than methane due to the presence of stronger dipole-dipole forces and hydrogen bonding. Water will have the highest boiling point due to its strong hydrogen bonding network.
Example 2: Compare the boiling points of propane (CH₃CH₂CH₃), butane (CH₃CH₂CH₂CH₃), and 2-methylpropane (isobutane, (CH₃)₃CH).
- Propane, Butane, and 2-Methylpropane: All are nonpolar and exhibit only LDFs.
Conclusion: Butane will have the highest boiling point because it's the largest molecule and has the strongest LDFs. Propane will have a higher boiling point than 2-methylpropane because it's less branched and has a greater surface area for intermolecular contact. 2-methylpropane will have the lowest boiling point.
Example 3: Compare the boiling points of chloromethane (CH₃Cl) and methane (CH₄).
- Chloromethane (CH₃Cl): Polar, exhibiting LDFs and dipole-dipole forces.
- Methane (CH₄): Nonpolar, only LDFs are present.
Conclusion: Chloromethane will have the higher boiling point because it possesses stronger dipole-dipole forces in addition to LDFs, compared to methane which only experiences LDFs.
Example 4: Compare the boiling points of dimethyl ether (CH₃OCH₃) and ethanol (CH₃CH₂OH).
- Dimethyl ether (CH₃OCH₃): Polar, exhibits LDFs and dipole-dipole forces.
- Ethanol (CH₃CH₂OH): Polar, exhibits LDFs, dipole-dipole forces, and hydrogen bonding.
Conclusion: Ethanol will have a significantly higher boiling point because of its strong hydrogen bonding, despite both molecules having similar molecular weights and both being polar.
Factors Beyond Intermolecular Forces
While intermolecular forces are the primary determinant of boiling point, other factors can play a minor role:
- Molecular weight: Generally, larger molecules with higher molecular weights have stronger LDFs and higher boiling points.
- Molecular shape: Linear molecules tend to have higher boiling points than branched molecules with the same molecular formula because of increased surface area for intermolecular interactions.
- Branching: Branching reduces surface area, decreasing the strength of LDFs and lowering the boiling point.
Conclusion
Predicting which substance has the lowest boiling point requires a systematic approach involving the identification and comparison of intermolecular forces. Understanding the relative strengths of LDFs, dipole-dipole forces, and hydrogen bonding, along with considerations of molecular size, shape, and branching, allows for accurate predictions of boiling point trends. Remember that hydrogen bonding is the strongest, followed by dipole-dipole interactions, and lastly, London Dispersion Forces. By carefully analyzing these factors, you can confidently determine which substance from a given set will exhibit the lowest boiling point. The examples provided offer a practical framework for applying these principles to various molecules and scenarios.
Latest Posts
Latest Posts
-
Viral Capsids Are Made From Subunits Called
Mar 15, 2025
-
A Negatively Charged Ion Is Called
Mar 15, 2025
-
Two Different Isotopes Of An Element Have Different
Mar 15, 2025
-
If A Pea Plant Shows A Recessive Phenotype
Mar 15, 2025
-
Is A Patent A Current Asset
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Has The Lowest Boiling Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
