Viral Capsids Are Made From Subunits Called
News Leon
Mar 15, 2025 · 7 min read
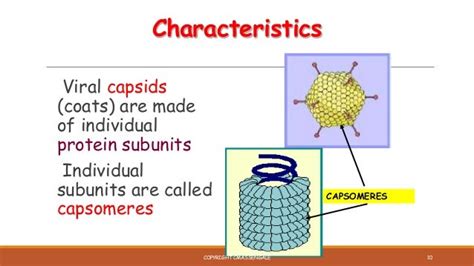
Table of Contents
Viral Capsids: Constructed from Subunits Called Capsomers
Viruses, those fascinatingly complex and sometimes devastatingly simple biological entities, rely on their capsids for survival and propagation. These protein shells, far from being monolithic structures, are meticulously assembled from numerous smaller subunits known as capsomers. Understanding the structure, assembly, and function of these capsomers is crucial to comprehending viral life cycles, developing antiviral therapies, and even exploring novel nanotechnological applications. This article delves deep into the world of viral capsids, exploring the intricacies of their capsomer components.
What are Capsids?
Before diving into the specifics of capsomers, let's establish a foundational understanding of capsids themselves. A capsid is the protein coat that encloses the viral genome, protecting it from degradation and facilitating its delivery into host cells. The capsid's structure is critical for viral infectivity, determining how the virus interacts with host cells, attaches to receptors, and releases its genetic material. The capsid's architecture varies considerably across different virus families, exhibiting remarkable diversity in size, shape, and symmetry.
Types of Viral Capsid Symmetry
Viral capsids are broadly categorized based on their symmetry:
-
Helical Symmetry: In viruses with helical symmetry, the capsomers arrange themselves in a spiral, forming a rod-shaped or filamentous capsid. Tobacco mosaic virus is a classic example. The length of the capsid is determined by the length of the viral genome, while the width is dictated by the dimensions of the capsomer subunits.
-
Icosahedral Symmetry: This is the most common type of capsid symmetry, exhibiting 20 triangular faces, 30 edges, and 12 vertices. Icosahedral symmetry provides an efficient way to enclose a large volume with a minimal number of capsomers. Many important human viruses, including adenoviruses and herpesviruses, possess icosahedral capsids.
-
Complex Symmetry: Some viruses, such as bacteriophages, have complex capsids that combine features of both helical and icosahedral symmetry. They often possess a head (icosahedral) and a tail (helical or complex) structure, enabling efficient attachment and genome delivery into the host bacterium.
Capsomers: The Building Blocks of Capsids
Now, let's turn our attention to the fundamental building blocks of these diverse capsid structures: the capsomers. These are individual protein subunits, often composed of multiple protein molecules (protomers), which self-assemble to form the complete capsid. The precise number and arrangement of capsomers dictate the overall shape and size of the capsid.
The Chemistry of Capsomers
Capsomers are predominantly composed of proteins, although some viruses might incorporate other molecules, such as lipids or carbohydrates, into their capsids. The amino acid sequence of the capsomer proteins is dictated by the viral genome, determining their specific three-dimensional structure and interactions with other capsomers during assembly. These interactions are crucial for the stability and functionality of the capsid. The specific amino acid residues involved in these interactions are often conserved across related viruses, reflecting the importance of these interactions for viral viability.
Different Types of Capsomers
Not all capsomers are created equal. Within a single capsid, different types of capsomers might exist, playing specialized roles in the overall structure and function. For example:
-
Pentons: Capsomers located at the 12 vertices of an icosahedral capsid. They are usually formed by five protomers, playing a vital role in defining the overall geometry and stability of the structure.
-
Hexons: Capsomers that form the 20 triangular faces of an icosahedral capsid. Each hexon is typically composed of six protomers. They are crucial in determining the size and surface area of the capsid.
The specific composition and arrangement of pentons and hexons determine the precise architecture of the icosahedral capsid. The precise ratio and arrangement of these capsomers is often highly conserved across viruses within a specific family, reflecting the essential role of these structural elements in the virus life cycle.
Capsomers and Viral Assembly
The assembly of viral capsids is a fascinating process, a delicate dance of self-organization. Capsomers, often initially present as separate units, spontaneously associate to form larger aggregates, eventually culminating in the complete, infectious capsid. This assembly process is highly specific and efficient, relying on a variety of weak non-covalent interactions, including:
-
Hydrophobic interactions: These interactions contribute significantly to the stability of the capsid by burying hydrophobic residues in the protein core.
-
Hydrogen bonds: These bonds play a crucial role in stabilizing the tertiary structure of individual capsomers and the interactions between them.
-
Electrostatic interactions: Attractive and repulsive forces between charged amino acid residues contribute to the assembly process and the overall stability of the capsid.
The assembly pathway can be quite complex, often involving chaperone proteins that assist in the correct folding and assembly of capsomers. Mutations that disrupt these interactions can severely compromise capsid assembly and viral infectivity, highlighting the importance of these interactions for viral viability.
Capsomers and Viral Entry into Host Cells
Once the virus has been assembled, the capsid plays a critical role in the virus's entry into the host cell. Specific proteins within the capsomers interact with receptors on the host cell surface, initiating the infection process. This interaction is often highly specific, with viruses exhibiting tropism for particular cell types or tissues.
The subsequent stages of viral entry involve conformational changes within the capsid, facilitating the release of the viral genome into the host cell cytoplasm. These changes can be triggered by a variety of factors, including pH changes in the endosome or the interaction with proteases within the host cell.
Understanding the interaction between capsomers and host cell receptors is a crucial aspect of antiviral drug development. Drugs that interfere with this interaction can effectively block viral entry, thereby preventing infection.
Capsomers in Viral Evolution
The evolution of viral capsids is a dynamic process, shaped by selective pressures exerted by the host immune system and environmental factors. Mutations in the capsomer genes can lead to changes in capsid structure, affecting the virus's ability to infect host cells and evade the immune response. This process drives the emergence of new viral strains and the adaptation of existing viruses to new hosts.
The high mutation rate of many RNA viruses, coupled with the importance of capsid proteins for infectivity, means that viral capsids can evolve rapidly, making it challenging to develop long-lasting antiviral treatments. This evolutionary arms race between viruses and their hosts shapes the landscape of viral pathogenesis and highlights the importance of understanding the role of capsomers in this dynamic interplay.
Capsomers: Potential Applications in Nanotechnology
The highly ordered and self-assembling nature of viral capsids makes them attractive candidates for nanotechnology applications. Their ability to encapsulate genetic material and other molecules, coupled with their biocompatibility and relatively easy manipulation, offer exciting possibilities for:
-
Drug delivery: Viral capsids can be engineered to deliver therapeutic molecules to specific cells or tissues, offering a promising approach to targeted drug delivery. By modifying the capsomer surface to target specific receptors, researchers can ensure delivery to the intended cells, maximizing therapeutic efficacy and minimizing side effects.
-
Biosensors: The sensitivity of capsid proteins to their environment can be exploited to develop highly sensitive biosensors. These sensors can detect changes in pH, temperature, or the presence of specific molecules, with potential applications in environmental monitoring, diagnostics, and therapeutics.
-
Vaccine development: Viral capsids have been increasingly utilized as platforms for vaccine development. By presenting viral antigens on the capsid surface, researchers can trigger a robust immune response, protecting against subsequent infection.
The research into the use of viral capsids in nanotechnology is rapidly expanding, revealing increasingly sophisticated applications and technologies based on these fundamental viral components. This promising field is at the forefront of innovative approaches in medicine, diagnostics, and beyond.
Conclusion: The Ever-Evolving World of Viral Capsomers
Viral capsids, constructed from the meticulously arranged subunits called capsomers, are far more than mere protective coats; they are sophisticated molecular machines driving viral life cycles. The remarkable diversity in capsid structure, assembly mechanisms, and interactions with host cells underscores the evolutionary success of viruses. Continued research into the intricate details of capsomer structure, assembly, and function is not only fundamental to our understanding of virology but also holds immense promise for revolutionary advancements in nanotechnology, therapeutics, and diagnostics. The self-assembly capabilities and biocompatibility of capsomers pave the way for groundbreaking applications in drug delivery systems, biosensors, and vaccines, transforming healthcare and other related fields. The exploration of the world of viral capsomers is an ongoing journey, filled with fascinating discoveries and immense potential for future innovation.
Latest Posts
Latest Posts
-
How Many Minutes Are There In One Day
Mar 15, 2025
-
A Sphere Has How Many Vertex
Mar 15, 2025
-
What Is The Electron Configuration For Ne
Mar 15, 2025
-
Tool Used To Detect Electric Charge
Mar 15, 2025
-
How Many Atoms Are In A Single Molecule Of H2o
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Viral Capsids Are Made From Subunits Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
