Which Is Not A Property Of An Ideal Gas
News Leon
Mar 20, 2025 · 6 min read
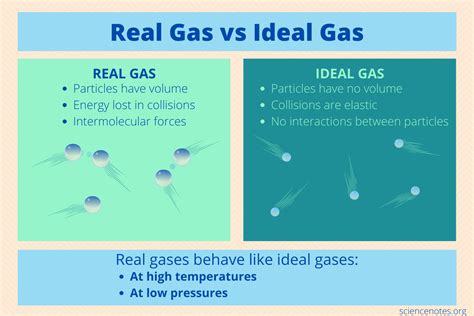
Table of Contents
- Which Is Not A Property Of An Ideal Gas
- Table of Contents
- Which Is Not a Property of an Ideal Gas? Delving into the Deviations from Perfection
- Understanding the Ideal Gas Model: A Foundation
- Properties Not Found in Ideal Gases: The Reality of Real Gases
- 1. Significant Intermolecular Forces
- 2. Non-Negligible Molecular Volume
- 3. Non-Elastic Collisions
- 4. Dependence on Temperature and Pressure
- 5. Non-Zero Internal Energy from Intermolecular Forces
- 6. Compressibility Factor (Z) Deviation from Unity
- Understanding the van der Waals Equation: A More Realistic Model
- Practical Implications of Non-Ideal Gas Behavior
- Conclusion: The Importance of Considering Reality
- Latest Posts
- Latest Posts
- Related Post
Which Is Not a Property of an Ideal Gas? Delving into the Deviations from Perfection
The ideal gas law, PV = nRT, is a cornerstone of chemistry and physics. It elegantly describes the relationship between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R). However, it's crucial to understand that the "ideal gas" is a theoretical construct. Real gases deviate from ideal behavior, particularly under conditions of high pressure and low temperature. This article will delve into the properties not characteristic of an ideal gas, exploring the reasons behind these deviations and their practical implications.
Understanding the Ideal Gas Model: A Foundation
Before exploring the imperfections, let's briefly revisit the assumptions underpinning the ideal gas model:
- Negligible intermolecular forces: Ideal gas molecules are assumed to have no attractive or repulsive forces between them. This means they interact only through perfectly elastic collisions.
- Negligible molecular volume: The volume occupied by the gas molecules themselves is considered negligible compared to the total volume of the container. The molecules are essentially point masses.
- Random motion and elastic collisions: Gas molecules are in constant, random motion, and collisions between molecules and the container walls are perfectly elastic (no energy loss).
- No internal energy from intermolecular forces: The total internal energy of the gas is solely due to the kinetic energy of the molecules. There's no contribution from potential energy associated with intermolecular interactions.
These assumptions simplify the behavior of gases, leading to the concise and useful ideal gas law. However, real gases deviate from this simplified model because these assumptions don't hold true under all conditions.
Properties Not Found in Ideal Gases: The Reality of Real Gases
Several properties directly contradict the assumptions of the ideal gas model, signifying the divergence of real gases from ideal behavior. Let's examine these in detail:
1. Significant Intermolecular Forces
Unlike ideal gases, real gases exhibit significant intermolecular forces. These forces, categorized as van der Waals forces (including London dispersion forces, dipole-dipole interactions, and hydrogen bonding), significantly influence the behavior of real gases, especially at high pressures and low temperatures.
- Attractive forces: At lower temperatures, the kinetic energy of gas molecules is reduced. Attractive forces become more dominant, causing molecules to cluster together, reducing the effective volume available for the gas to occupy. This leads to a lower pressure than predicted by the ideal gas law.
- Repulsive forces: At high pressures, the molecules are forced closer together. Repulsive forces between them become significant, leading to a higher pressure than predicted by the ideal gas law. The molecules are occupying a larger portion of the total volume.
2. Non-Negligible Molecular Volume
Ideal gas molecules are treated as point masses with negligible volume. However, real gas molecules possess a finite volume. At high pressures, this molecular volume becomes a significant fraction of the total volume, causing deviations from the ideal gas law. The available volume for the gas to occupy is less than the total volume of the container. This leads to a higher pressure than predicted.
3. Non-Elastic Collisions
While ideal gas collisions are perfectly elastic (no energy loss), real gas collisions are not perfectly elastic. Some kinetic energy is lost during collisions, converting into vibrational or rotational energy within the molecules. This energy loss affects the overall pressure and temperature of the gas.
4. Dependence on Temperature and Pressure
The ideal gas law implies that the behavior of a gas is independent of temperature and pressure. However, real gases show a marked dependence on both. As mentioned earlier, at low temperatures, intermolecular attractive forces become more pronounced, while at high pressures, molecular volume and repulsive forces become more important.
5. Non-Zero Internal Energy from Intermolecular Forces
Ideal gases have internal energy solely from the kinetic energy of their molecules. In contrast, real gases possess additional internal energy due to intermolecular potential energy. This potential energy arises from the attractive and repulsive forces between the molecules and influences the gas's thermodynamic properties.
6. Compressibility Factor (Z) Deviation from Unity
The compressibility factor (Z) is a measure of how much a real gas deviates from ideal gas behavior. It's defined as Z = PV/nRT. For an ideal gas, Z = 1. However, for real gases, Z deviates from unity, reflecting the influence of intermolecular forces and molecular volume. At low pressures, Z is usually less than 1 due to attractive forces, while at high pressures, Z is often greater than 1 due to repulsive forces.
Understanding the van der Waals Equation: A More Realistic Model
The van der Waals equation is a more sophisticated model that accounts for intermolecular forces and molecular volume:
(P + a(n/V)²)(V - nb) = nRT
Where:
- 'a' represents the attractive forces between molecules.
- 'b' represents the volume occupied by the molecules.
The van der Waals equation provides a better approximation of real gas behavior than the ideal gas law, particularly under conditions where the ideal gas law fails. However, even the van der Waals equation is not a perfect representation of all real gases, as it simplifies the complex interactions between molecules. More complex equations of state are needed for highly accurate predictions under extreme conditions.
Practical Implications of Non-Ideal Gas Behavior
Understanding the deviations from ideal gas behavior is crucial in numerous applications:
- Chemical engineering: Accurate modeling of real gas behavior is essential for designing and optimizing chemical processes, particularly those involving high pressures and temperatures, such as in the petrochemical industry.
- Refrigeration and air conditioning: Understanding the behavior of refrigerants under various pressure and temperature conditions is vital for designing efficient and safe refrigeration and air conditioning systems.
- Environmental science: Modeling the behavior of atmospheric gases accurately is crucial for understanding climate change and other atmospheric phenomena.
- Materials science: Understanding the behavior of gases in materials is important for designing and manufacturing advanced materials.
Conclusion: The Importance of Considering Reality
While the ideal gas law provides a useful simplification of gas behavior, it's crucial to remember that it's an idealized model. Real gases deviate from ideal behavior, especially under conditions of high pressure and low temperature. Understanding the properties not found in ideal gases – significant intermolecular forces, non-negligible molecular volume, non-elastic collisions, dependence on temperature and pressure, non-zero internal energy from intermolecular forces, and compressibility factor deviation – is essential for accurate modeling and predictions in various scientific and engineering applications. More sophisticated models, such as the van der Waals equation, provide better approximations but still fall short of perfectly capturing the intricate behavior of real gases under all conditions. The ongoing quest for more accurate and comprehensive models reflects the complexity and fascinating nature of the gaseous state.
Latest Posts
Latest Posts
-
Which Of The Following Statements About Surface Tension Is False
Mar 22, 2025
-
How Does Lymph Differ From Plasma
Mar 22, 2025
-
Ch3 Ch2 Ch Ch Ch2 Ch2 Ch3 Name
Mar 22, 2025
-
Which Of The Following Best Describes A Population
Mar 22, 2025
-
Energy Level Diagram Of Hydrogen Atom
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Which Is Not A Property Of An Ideal Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
