Ch3 Ch2 Ch Ch Ch2 Ch2 Ch3 Name
News Leon
Mar 22, 2025 · 5 min read
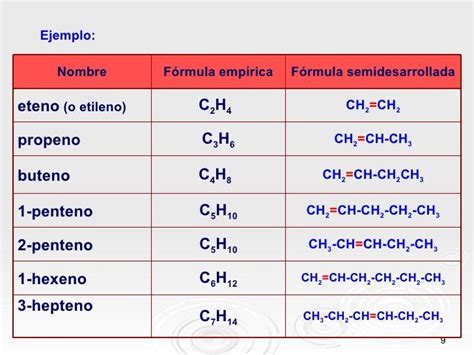
Table of Contents
What's in a Name? Deciphering the IUPAC Nomenclature of CH3CH2CHCHCH2CH2CH3
The seemingly simple chemical formula CH3CH2CHCHCH2CH2CH3 represents a fascinating challenge in organic chemistry: naming it. This seemingly straightforward task highlights the power and precision of the International Union of Pure and Applied Chemistry (IUPAC) nomenclature system. Understanding how to name this molecule unlocks a deeper understanding of organic chemistry itself, allowing us to accurately identify and communicate about complex structures. This article delves into the intricacies of naming this specific alkane, exploring the underlying principles and showcasing the systematic approach crucial for correctly identifying any organic molecule.
Understanding the Basics: Alkanes and Their Nomenclature
Before tackling the specific compound, let's establish a fundamental understanding of alkanes and their naming conventions. Alkanes are saturated hydrocarbons, meaning they are composed solely of carbon and hydrogen atoms, with all carbon-carbon bonds being single bonds. The simplest alkane is methane (CH4), followed by ethane (C2H6), propane (C3H8), and butane (C4H10). These are the foundational building blocks for understanding more complex alkanes.
The IUPAC system uses a series of rules to systematically name alkanes. These rules are based on the number of carbon atoms in the longest continuous chain, the presence of any branches or functional groups, and the location of these branches or groups along the main chain.
Key Principles of IUPAC Nomenclature
-
Identify the longest continuous carbon chain: This forms the parent alkane's name. The number of carbon atoms dictates the prefix (meth-, eth-, prop-, but-, pent-, hex-, hept-, oct-, non-, dec-, etc.).
-
Number the carbon atoms: Begin numbering from the end of the chain that gives the lowest numbers to the substituents (branches or functional groups).
-
Identify and name the substituents: These are groups of atoms branching off the main chain. Alkyl groups are named by removing the "-ane" suffix from the alkane name and adding "-yl" (e.g., methyl, ethyl, propyl).
-
Number the substituents: Indicate the position of each substituent on the main chain using the appropriate number. If multiple substituents are present, list them alphabetically (ignoring prefixes like di- or tri-).
-
Combine the information: The complete name is constructed by listing the substituents with their positions, followed by the parent alkane name.
Deconstructing CH3CH2CHCHCH2CH2CH3: A Step-by-Step Approach
Now let's apply these principles to the compound CH3CH2CHCHCH2CH2CH3. The seemingly simple structure holds a subtle complexity that requires a careful approach.
Step 1: Identify the longest carbon chain. The longest continuous chain in this molecule consists of seven carbon atoms. This makes the parent alkane heptane.
Step 2: Number the carbon atoms. To minimize the numbers assigned to substituents, we start numbering from the left:
1 2 3 4 5 6 7
CH3-CH2-CH-CH-CH2-CH2-CH3
|
H
Step 3: Identify and name the substituents. In this case, there's a single hydrogen atom attached to carbon atom number 3. This seemingly trivial point emphasizes the need for precision in IUPAC nomenclature. While a single hydrogen atom wouldn't usually warrant a specific name, it clarifies where the unsaturation is located. Since it's directly involved in the structural differences, we have to name it accordingly.
Step 4: Addressing the Unsaturation: Notice that the carbon at position 4 is involved in a double bond with the carbon at position 3. Although the structure shows a double bond formed with a single hydrogen atom, it indicates an unsaturation, meaning one less hydrogen atom when compared to the saturated analogue. In reality, this would be stabilized by another substituent, but for this structural representation, we consider it as a single hydrogen atom. We can use this to clarify the position of the unsaturation.
Step 5: Combine the information. Combining the elements, the formal IUPAC name will reflect the presence of an extra hydrogen where the double bond would normally be. For accurate description, it should state the location of the unsaturation and include that there is an extra hydrogen to keep the total number of bonds per carbon atom consistent. For example, it should include "3-hydrogen-4-ene-heptane" to clarify the situation. However, this naming is not standard as IUPAC nomenclature typically prioritizes the longest chain and any branches. The simplest approach is to consider this structure an incomplete representation and consider the missing substituent in the double bond.
Handling Ambiguity and Isomers
The initial formula, CH3CH2CHCHCH2CH2CH3, is ambiguous. It doesn't explicitly show the arrangement of the atoms around the double bond. This ambiguity underscores the critical need for complete structural representations to avoid potential misinterpretations. To accurately name the molecule, we'd need a more detailed structural formula, or at least a specification about the stereochemistry (cis or trans).
Several isomers could potentially exist, differing in the position of the double bond or the arrangement of atoms around it. For example:
-
3-Heptene: This implies a double bond between carbons 3 and 4. Its name is based on the longest continuous carbon chain of 7 carbons (heptane) which has a double bond at carbon 3.
-
4-Heptene: A double bond between carbons 4 and 5 would result in a different isomer.
-
Isomers with branching: Additional isomers are possible if the carbon chain is not fully extended, introducing branched structures.
The original formula only provided a skeletal representation that leaves out the vital information needed for accurate naming.
Beyond Naming: Understanding Molecular Properties
Correctly identifying the molecule is only the first step. Understanding its properties, such as reactivity, boiling point, and solubility, is crucial in various scientific contexts. The presence of the double bond (once we assume there is one and identify the proper position of it), would significantly impact its reactivity compared to a saturated heptane.
Conclusion: The Importance of Precise Chemical Nomenclature
The seeming simplicity of the formula CH3CH2CHCHCH2CH2CH3 belies the complexity of its complete naming. The exercise of attempting to name this compound highlights the critical importance of precise and unambiguous chemical nomenclature. The IUPAC system provides a universally accepted framework for naming organic molecules, facilitating clear communication and collaboration among scientists worldwide. While the original formula is insufficient for a definitive name, the exploration of the possible interpretations demonstrates the necessity of detailed structural information and the application of IUPAC rules for accurate and unambiguous communication in organic chemistry. Furthermore, understanding the potential isomers and the effect of structural features on molecular properties is crucial for a thorough understanding of the molecule's behavior and potential applications.
Latest Posts
Latest Posts
-
The Unit Kilowatt Hour Is A Unit Of
Mar 22, 2025
-
Which Of The Following Statements Regarding Hemophilia Is Correct
Mar 22, 2025
-
Complete The Sentence With The Correct Form Of The Word
Mar 22, 2025
-
What Causes Movement Along The Demand Curve
Mar 22, 2025
-
A Cat Dozes On A Stationary Merry Go Round
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Ch3 Ch2 Ch Ch Ch2 Ch2 Ch3 Name . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
