Which Elements Have Complete Outer Shells
News Leon
Mar 31, 2025 · 6 min read
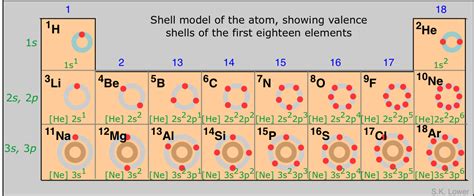
Table of Contents
Which Elements Have Complete Outer Shells? Understanding Octet Rule and Noble Gases
The quest to understand the behavior of elements and their interactions has been a central theme in chemistry. A key concept in this understanding is the octet rule, which dictates that atoms tend to gain, lose, or share electrons in order to achieve a full outer electron shell, resembling the stable electron configuration of noble gases. This article delves into the fascinating world of elements with complete outer shells, exploring their unique properties and the implications for chemical bonding.
What is a Complete Outer Shell?
Before we dive into which elements possess this coveted full outer shell, let's clarify what we mean. Atoms are composed of a nucleus containing protons and neutrons, surrounded by electrons orbiting in distinct energy levels or shells. The outermost shell, also known as the valence shell, plays a crucial role in determining an element's reactivity. A complete outer shell signifies that this valence shell is filled to its maximum capacity with electrons. This state of electron configuration provides exceptional stability, making these atoms less likely to participate in chemical reactions.
The Noble Gases: The Perfect Examples
The most prominent examples of elements with complete outer shells are the noble gases, also known as inert gases. Located in Group 18 (VIIIA) of the periodic table, these elements include Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), Radon (Rn), and the synthetically created Oganesson (Og). Their characteristic feature is their exceptional stability due to their filled valence shells.
Helium (He): A Unique Case
Helium, the lightest noble gas, stands apart slightly. Its outermost shell, the 1s orbital, can only accommodate two electrons. Unlike other noble gases that follow the octet rule (eight electrons in the valence shell), helium achieves stability with only two electrons, fulfilling its shell capacity. This exception highlights that the drive towards complete outer shells isn't always about eight electrons, but rather achieving a stable configuration.
Neon (Ne), Argon (Ar), and Others: The Octet Rule in Action
Neon, argon, and the rest of the noble gases (excluding helium) follow the octet rule, possessing eight electrons in their outermost shell. This full complement of electrons creates an exceptionally stable electron configuration, making them extremely unreactive. They rarely form chemical bonds with other elements, contributing to their classification as "inert" gases. This inherent stability is a direct consequence of their complete outer electron shells.
Implications of Complete Outer Shells: Chemical Inertness
The significance of a complete outer shell lies in its impact on an element's chemical behavior. Elements strive for stability, and achieving a complete outer shell is a powerful way to attain this. Because noble gases already have complete outer shells, they don't readily lose, gain, or share electrons to form chemical bonds. This accounts for their remarkably low reactivity and explains why they are often found as monatomic gases – individual atoms, not bonded together.
Exceptions to the Octet Rule: When the Rules Bend
While the octet rule is a useful guideline, it's not without exceptions. Some elements can form stable compounds even without having a complete octet in their valence shell. These exceptions often involve elements in the second row (period) of the periodic table, like beryllium, boron, and aluminum.
Electron Deficiency: Beryllium and Boron
Beryllium (Be) and boron (B) are often electron-deficient, meaning their valence shells don't contain a full octet in some compounds. For example, beryllium can form covalent bonds with only four electrons in its valence shell, while boron often participates in compounds with only six valence electrons. These exceptions showcase the limitations of the octet rule as an absolute principle.
Expanded Octet: Elements Beyond the Third Period
Elements in the third period and beyond can have more than eight electrons in their valence shells. This is because their valence shells can accommodate electrons in d orbitals, which are not available to elements in the second period. Phosphorus, sulfur, and other elements in this and subsequent periods can exhibit expanded octets in certain compounds.
Hypervalence: A Deeper Dive
The phenomenon of having more than eight valence electrons is known as hypervalence. This occurs when elements use their d orbitals to accommodate additional electrons beyond the standard octet. This expands the possibilities for chemical bonding and allows for the formation of compounds that wouldn't be predicted based solely on the octet rule.
The Role of Complete Outer Shells in Chemical Bonding
The drive towards a complete outer shell is a fundamental driving force behind chemical bonding. Atoms react with each other to achieve this stable configuration. There are different types of chemical bonds:
Ionic Bonding: Electron Transfer
In ionic bonding, atoms transfer electrons to achieve a complete outer shell. Typically, a metal atom loses electrons (becoming a positively charged cation), and a nonmetal atom gains these electrons (becoming a negatively charged anion). The electrostatic attraction between these oppositely charged ions forms the ionic bond. Sodium chloride (NaCl) is a classic example; sodium loses an electron to achieve a full outer shell, while chlorine gains an electron to do the same.
Covalent Bonding: Electron Sharing
Covalent bonding involves the sharing of electrons between atoms to achieve a complete outer shell for each atom. This type of bonding is common among nonmetal atoms. Water (H₂O) is a prime example; oxygen shares electrons with two hydrogen atoms to complete its octet, while each hydrogen atom shares its electron with oxygen to fill its own shell (albeit with only two electrons).
Beyond the Octet Rule: A Broader Perspective
While the octet rule provides a valuable framework for understanding chemical bonding, it's important to recognize its limitations. The concept of complete outer shells, while crucial, isn't the sole determinant of chemical behavior. Other factors, such as electronegativity, atomic size, and the presence of d and f orbitals, significantly influence the reactivity and bonding patterns of elements. Furthermore, the octet rule is primarily concerned with the main group elements; the transition metals and inner transition metals exhibit different bonding patterns and behaviors.
Conclusion: The Importance of Outer Shell Electrons
Understanding which elements have complete outer shells and the implications of this configuration is vital for comprehending the fundamentals of chemistry. The noble gases, with their stable electron configurations, serve as benchmarks for chemical inertness. However, the octet rule, while a helpful guideline, possesses exceptions, leading to a richer and more nuanced understanding of chemical bonding and reactivity. This detailed exploration of complete outer shells reinforces the interconnectedness of atomic structure, chemical bonding, and the behavior of matter. The study of these principles forms the bedrock of chemical understanding and fuels continued advancements in chemistry and related fields.
Latest Posts
Latest Posts
-
All Of The Following Characteristics Are Associated With Epithelium Except
Apr 02, 2025
-
This Organelle Pumps Out Excess Water
Apr 02, 2025
-
If The Demand For A Good Is Elastic Then
Apr 02, 2025
-
Solid Liquid Or Gas That A Wave Travels Through
Apr 02, 2025
-
What Is The Antiderivative Of E 2x
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Elements Have Complete Outer Shells . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
