Which Element Has Lowest Ionization Energy
News Leon
Mar 28, 2025 · 5 min read
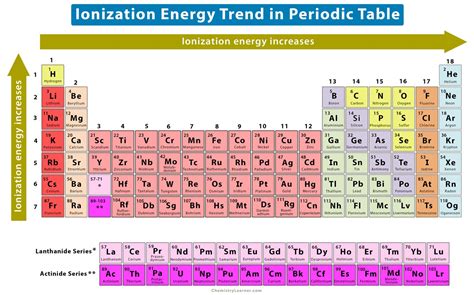
Table of Contents
Which Element Has the Lowest Ionization Energy? Unveiling the Secrets of Francium
The quest to identify the element with the lowest ionization energy is a fascinating journey into the heart of atomic structure and periodic trends. Ionization energy, the energy required to remove an electron from a gaseous atom, is a fundamental property reflecting an element's electronegativity and reactivity. Understanding this property helps us predict chemical behavior and explore the mysteries of the periodic table. This comprehensive article delves into the answer, explaining the underlying principles and exploring the unique characteristics of the element holding the record.
Understanding Ionization Energy
Before we pinpoint the element with the lowest ionization energy, let's solidify our understanding of the concept itself. Ionization energy is not a single value but rather a series of values, representing the energy required to remove successive electrons. The first ionization energy (IE₁) is the energy needed to remove the outermost electron, the second ionization energy (IE₂) is the energy required to remove the next electron, and so on. These values increase progressively as it becomes increasingly difficult to remove electrons from an increasingly positively charged ion.
Factors Influencing Ionization Energy
Several factors govern an element's ionization energy. These factors are intricately linked to the atom's electronic structure and its position within the periodic table. The key factors include:
-
Atomic Radius: Larger atoms have larger atomic radii, meaning their outermost electrons are farther from the nucleus. The weaker electrostatic attraction between the nucleus and these distant electrons results in lower ionization energies.
-
Nuclear Charge: A higher nuclear charge (more protons) leads to a stronger attraction for electrons, resulting in higher ionization energies.
-
Shielding Effect: Inner electrons shield the outermost electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the outermost electrons, lowering the ionization energy.
-
Electron Configuration: Electrons in filled subshells (e.g., s² or p⁶) are more stable and require more energy to remove than electrons in partially filled subshells. This is due to the stable electron arrangements associated with filled subshells, a concept directly related to Hund's rule and the Pauli exclusion principle.
The Periodic Trend: Ionization Energy Across the Table
As we move across the periodic table from left to right, the ionization energy generally increases. This is because the nuclear charge increases while the shielding effect remains relatively constant, leading to a stronger attraction for electrons. Conversely, as we move down a group (column) in the periodic table, the ionization energy generally decreases. This is attributed to the increasing atomic radius and enhanced shielding effect, which weaken the electrostatic attraction between the nucleus and the outermost electrons.
The Champion: Francium (Fr)
After considering these factors and observing the periodic trends, the answer becomes clear: Francium (Fr) possesses the lowest first ionization energy of all elements. This is a direct consequence of its position at the bottom of Group 1 (alkali metals) in the periodic table.
Why Francium Holds the Record
Francium's exceptionally low ionization energy stems from a combination of factors:
-
Largest Atomic Radius: Francium possesses the largest atomic radius of all known elements. Its outermost electron is extremely distant from the positively charged nucleus, resulting in a weak electrostatic attraction. This weak attraction makes it exceptionally easy to remove the electron.
-
High Shielding Effect: The numerous inner electron shells effectively shield the outermost electron from the nucleus's positive charge, further weakening the attraction.
-
Low Effective Nuclear Charge: The combination of large atomic radius and strong shielding leads to a significantly low effective nuclear charge experienced by the outermost electron. This significantly reduces the energy required for ionization.
The Challenges of Studying Francium
Studying francium's properties directly presents significant challenges due to its extreme radioactivity and short half-life. It's an extremely rare element, making large-scale experiments impractical. Most of our understanding of francium's properties is derived from theoretical calculations and extrapolations based on the trends observed in other alkali metals.
Comparison with Other Alkali Metals
While francium holds the title for the lowest ionization energy, it's helpful to compare it with other alkali metals in Group 1:
-
Cesium (Cs): Before the discovery and characterization of francium, cesium held the record for the lowest ionization energy. Its lower ionization energy compared to other alkali metals above it is explained by the same principles affecting francium: large atomic radius and increased shielding.
-
Rubidium (Rb), Potassium (K), Sodium (Na), Lithium (Li): As we move up Group 1, the atomic radius decreases, and the shielding effect weakens, resulting in a gradual increase in ionization energy.
Implications and Applications
While the practical applications of francium are extremely limited due to its radioactivity and scarcity, understanding its exceptionally low ionization energy contributes significantly to our understanding of fundamental atomic properties and periodic trends. This knowledge is crucial in various fields:
-
Theoretical Chemistry: Francium's properties provide valuable data for testing and refining theoretical models of atomic structure and chemical bonding.
-
Nuclear Physics: Francium's radioactive decay is studied in nuclear physics research.
-
Spectroscopy: Studying the spectral lines of francium contributes to our understanding of its electronic structure and energy levels.
Conclusion: A Triumph of Atomic Structure
The element with the lowest ionization energy is francium (Fr). This remarkable property is a direct consequence of its position in the periodic table, its large atomic radius, strong shielding effect, and consequently low effective nuclear charge. While its extreme radioactivity and scarcity limit its practical applications, the study of francium remains vital for furthering our fundamental understanding of atomic structure and chemical behavior. Its unique properties highlight the power of periodic trends in predicting and understanding the behavior of elements. The journey to understanding francium and its ionization energy is a testament to the elegance and interconnectedness of the principles governing the world of atoms and molecules.
Latest Posts
Latest Posts
-
Si Unit Of Density Of Water
Mar 31, 2025
-
What Is The Molecular Geometry Of Bef2
Mar 31, 2025
-
Attached Earlobes Is A Recessive Trait In Humans
Mar 31, 2025
-
How Many Electrons Does Nitrogen Have In Its Outer Shell
Mar 31, 2025
-
Which Electrolyte Is A Major Cation In Body Fluid
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has Lowest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
