What Is The Molecular Geometry Of Bef2
News Leon
Mar 31, 2025 · 5 min read
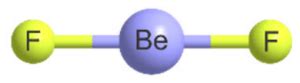
Table of Contents
What is the Molecular Geometry of BeF₂? A Deep Dive into Linear Structures
Beryllium difluoride (BeF₂) is a fascinating inorganic compound that provides a great example of how molecular geometry impacts a molecule's properties. Understanding its structure is crucial for comprehending its unique characteristics and behavior. This comprehensive article will delve into the molecular geometry of BeF₂, exploring the underlying principles of valence shell electron pair repulsion (VSEPR) theory, hybridization, and the resulting linear structure. We'll also examine the implications of this geometry on BeF₂'s physical and chemical properties.
Understanding VSEPR Theory: The Foundation of Molecular Geometry
The cornerstone of predicting molecular geometry is the valence shell electron pair repulsion (VSEPR) theory. This theory postulates that electron pairs in the valence shell of a central atom repel each other, and they arrange themselves to minimize this repulsion. This arrangement determines the molecule's overall shape. The repulsion between electron pairs follows this order: lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair.
In the case of BeF₂, beryllium (Be) is the central atom, and two fluorine (F) atoms are bonded to it. Beryllium is an alkaline earth metal with two valence electrons. Each fluorine atom contributes one electron to the bond, resulting in two bonding pairs and zero lone pairs on the beryllium atom.
Applying VSEPR to BeF₂: A Simple Linear Structure
With two bonding pairs and zero lone pairs around the central beryllium atom, the VSEPR theory predicts a linear molecular geometry for BeF₂. The two fluorine atoms are positioned 180° apart from each other, minimizing the repulsion between the bonding electron pairs. This creates a perfectly symmetrical molecule.
Hybridization in BeF₂: Exploring sp Orbitals
While VSEPR theory effectively predicts the molecular shape, understanding the orbital hybridization provides a deeper insight into the bonding mechanism. In BeF₂, the beryllium atom undergoes sp hybridization.
The Role of sp Hybridization: Creating Two Hybrid Orbitals
In its ground state, beryllium has a 1s²2s² electron configuration. However, to form two covalent bonds with the fluorine atoms, it needs two unpaired electrons. This is achieved through promotion, where one electron from the 2s orbital is promoted to the empty 2p orbital, resulting in a 1s²2s¹2p¹ configuration. These two unpaired electrons then hybridize to form two sp hybrid orbitals.
The Formation of Sigma Bonds: Overlap of sp and p Orbitals
Each of these sp hybrid orbitals on the beryllium atom overlaps with a p orbital from each fluorine atom, forming two sigma (σ) bonds. These sigma bonds are strong and contribute to the stability of the BeF₂ molecule. The linear arrangement is a direct consequence of the orientation of these sp hybrid orbitals, which are positioned 180° apart.
Delving Deeper: Properties Arising from the Linear Geometry of BeF₂
The linear geometry of BeF₂ significantly influences its physical and chemical properties.
Bond Angle and Dipole Moment: A Perfectly Symmetrical Molecule
The bond angle in BeF₂ is 180°. This perfect linearity is a direct consequence of the sp hybridization and the minimal electron-pair repulsion. Due to this symmetry, the individual bond dipoles cancel each other out, resulting in a zero dipole moment for BeF₂. This means that BeF₂ is a nonpolar molecule despite the difference in electronegativity between beryllium and fluorine.
Physical Properties: A Volatile and Low-Melting Point Compound
BeF₂ is a volatile compound, meaning it easily transitions from a solid to a gas phase. Its relatively low melting point is also attributable to its linear structure. The weak intermolecular forces between the nonpolar BeF₂ molecules result in less energy being required to overcome these forces during melting.
Chemical Reactivity: A Lewis Acid Character
Beryllium, being an electron-deficient species, is a strong Lewis acid. It readily accepts electron pairs to achieve a more stable electron configuration. The linear geometry of BeF₂ allows for easy access to the electron-deficient beryllium atom, making it highly reactive towards Lewis bases like water and ammonia. These Lewis bases can donate their electron pairs to the empty orbitals on the beryllium atom, forming adducts.
BeF₂ in Different Phases: Maintaining the Linear Geometry
The linear molecular geometry of BeF₂ is generally maintained across different phases. While intermolecular forces can influence the packing of molecules in the solid and liquid phases, the intramolecular bonding within each BeF₂ molecule remains largely unaffected and retains its linear structure. However, in the liquid phase and particularly in solutions, complexation with solvent molecules can influence the coordination geometry around beryllium to a certain extent, although the fundamental linear nature of the Be-F bond typically persists.
Comparing BeF₂ with Other Linear Molecules: Shared Characteristics
Numerous other molecules exhibit linear geometry, often due to similar bonding and hybridization characteristics. Examples include carbon dioxide (CO₂), hydrogen cyanide (HCN), and other diatomic molecules like oxygen (O₂) and nitrogen (N₂). All of these molecules have a central atom with two bonding pairs and zero lone pairs, leading to the predicted linear arrangement by VSEPR theory. The hybridization of the central atoms in these molecules is diverse, although it always results in two hybrid orbitals suitable for forming two sigma bonds, which are arranged linearly to minimize electron repulsion.
Practical Applications of BeF₂ and Its Geometry
The linear geometry of BeF₂ and the resulting properties have some applications, though it's not as widely used as other compounds. Historically, BeF₂ found use in certain specialized applications owing to its optical transparency and low refractive index. However, safety concerns related to beryllium toxicity have significantly limited its applications.
Conclusion: The Significance of Understanding Molecular Geometry
Understanding the molecular geometry of BeF₂, as elucidated by VSEPR theory and confirmed by experimental evidence, is crucial for interpreting its properties and potential applications. Its linear structure, arising from sp hybridization, directly influences its physical and chemical behavior, including its nonpolar nature, volatility, and Lewis acidity. By comparing BeF₂ to other molecules with similar geometry, we enhance our understanding of the fundamental principles governing molecular structure and its profound impact on the macroscopic properties of matter. This knowledge is fundamental in many areas of chemistry, materials science, and engineering. Further research continues to explore the intricacies of beryllium chemistry and its potential uses, keeping in mind the necessary precautions due to its toxicity.
Latest Posts
Latest Posts
-
Common Factors Of 8 And 36
Apr 01, 2025
-
The Needle On A Compass Always Points Towards What Direction
Apr 01, 2025
-
How Many Ounces In 1 8 Pound
Apr 01, 2025
-
How Many Elements Are There In The Sample Space
Apr 01, 2025
-
Ca Oh 2 Hcl Cacl2 H2o
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is The Molecular Geometry Of Bef2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
