Which Element Has 2 Valence Electrons
News Leon
Apr 01, 2025 · 5 min read
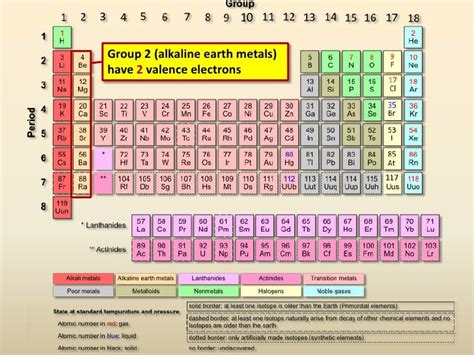
Table of Contents
Which Element Has 2 Valence Electrons? Exploring the Group 2 Alkaline Earth Metals
The question, "Which element has 2 valence electrons?" leads us into the fascinating world of electron configuration and the periodic table. While several elements can exhibit two valence electrons under specific circumstances, only one group consistently possesses this characteristic: the alkaline earth metals. This article will delve deep into this group, exploring their properties, chemical behavior, and the significance of their two valence electrons. We’ll also briefly touch upon exceptions and other elements that might temporarily show two valence electrons.
Understanding Valence Electrons
Before diving into specific elements, let's clarify the concept of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are crucial because they determine an element's chemical reactivity and bonding behavior. They are the electrons most likely to participate in chemical reactions, forming bonds with other atoms. The number of valence electrons largely dictates how many bonds an atom can form and what types of bonds it prefers (ionic, covalent, etc.).
The Alkaline Earth Metals: The Family with 2 Valence Electrons
The alkaline earth metals are located in Group 2 of the periodic table. This group includes:
- Beryllium (Be)
- Magnesium (Mg)
- Calcium (Ca)
- Strontium (Sr)
- Barium (Ba)
- Radium (Ra)
Each of these elements consistently has two electrons in its outermost electron shell, making them the definitive answer to our initial question. This commonality leads to striking similarities in their chemical properties and reactivity.
Chemical Properties and Reactivity Driven by Two Valence Electrons
The presence of two valence electrons profoundly influences the chemical behavior of alkaline earth metals. They readily lose these two electrons to achieve a stable electron configuration, resembling the noble gas in the previous period. This electron loss results in the formation of +2 cations.
-
Electropositivity: Alkaline earth metals are highly electropositive, meaning they have a strong tendency to lose electrons and form positive ions. This tendency increases as you move down the group. Beryllium, being at the top, is less reactive than heavier elements like radium.
-
Reactivity with Water and Acids: Their reactivity with water and acids is another key characteristic. While beryllium reacts only very slowly with water, the rest react more readily, producing hydrogen gas and the corresponding metal hydroxide. The reactions become increasingly vigorous as you descend the group. For example, calcium reacts moderately, whereas strontium and barium react more violently. Similarly, their reaction with acids produces hydrogen gas and a salt.
-
Oxidation States: Due to their two valence electrons, their most common oxidation state is +2. Higher oxidation states are extremely rare and generally occur in unusual chemical environments.
Applications and Uses
The properties stemming from their two valence electrons translate into numerous practical applications. Here are a few examples:
-
Magnesium (Mg): Widely used in lightweight alloys for automobiles, aircraft, and other applications. Its ability to form strong, yet lightweight, alloys is a direct consequence of its electronic structure.
-
Calcium (Ca): Essential for biological systems, playing a critical role in bone formation, muscle contraction, and nerve impulse transmission. Its +2 ionic form readily interacts with other biological molecules.
-
Strontium (Sr): Used in fireworks to produce a bright red color. The electronic transitions of strontium ions are responsible for this characteristic red emission.
-
Barium (Ba): Used in various industrial applications, including drilling muds, glass manufacturing, and the production of certain types of paints. Its reactivity and ability to form specific compounds are leveraged in these applications.
Beyond Group 2: Exceptions and Elements Exhibiting Two Valence Electrons in Specific Cases
While the alkaline earth metals are the definitive answer, certain elements can temporarily exhibit two valence electrons under particular circumstances. These are exceptions and don't represent their typical or most stable state:
-
Transition Metals: Some transition metals, located in the d-block of the periodic table, can exhibit variable oxidation states, including +2. This doesn't mean they have two valence electrons in their neutral state; rather, they lose electrons from multiple shells, often including valence electrons and d-electrons, to achieve the +2 oxidation state. Examples include Iron (Fe) and Copper (Cu), which can form Fe²⁺ and Cu²⁺ ions, respectively, but these are not their sole or most common oxidation states.
-
Post-transition Metals: Certain post-transition metals might also show a +2 oxidation state under specific conditions. This is generally less common and less predictable than in transition metals.
-
Excited States: In excited states, an electron can be promoted to a higher energy level, resulting in a temporary change in the number of valence electrons. However, these excited states are short-lived and generally return to the ground state quickly.
It is crucial to remember that the consistent and predictable presence of two valence electrons defines the alkaline earth metals. Other elements' ability to exhibit a +2 oxidation state is context-dependent and should not be interpreted as having two valence electrons in their fundamental state.
The Importance of Understanding Valence Electrons
Understanding valence electrons is fundamental to comprehending chemical bonding, reactivity, and the properties of elements. The number of valence electrons dictates how atoms interact with each other, forming molecules and compounds. This knowledge is crucial in diverse fields, from materials science and engineering to biology and medicine. The consistent two valence electrons of alkaline earth metals highlight the significant influence of electron configuration on an element's overall characteristics and utility.
Conclusion: A Comprehensive Look at Elements with Two Valence Electrons
The question of which element has two valence electrons has led us on a journey exploring the fundamentals of atomic structure and chemical behavior. While several elements can exhibit a +2 oxidation state under specific conditions, the consistent and defining characteristic of two valence electrons belongs to the alkaline earth metals (Group 2). Their properties, applications, and reactivity are all directly linked to this fundamental aspect of their electron configuration. Understanding this link is key to comprehending their importance in various scientific and technological fields. The consistent behavior of these elements offers a crucial insight into the power of electron configuration in shaping the behavior and properties of chemical elements. Further exploration into the periodic table and its organization will reveal more examples of how electron configuration dictates chemical properties and forms the basis of countless applications.
Latest Posts
Latest Posts
-
2 Is The Only Even Prime Number
Apr 02, 2025
-
Distance Between Earth And Moon In Light Years
Apr 02, 2025
-
Which Of The Following Is Chemically Inert Unreactive
Apr 02, 2025
-
The Standard Unit For Measuring Volume Is
Apr 02, 2025
-
Materials Like Rubber That Resist The Flow Of E
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has 2 Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
