What Type Of Mixture Is Oil And Water
News Leon
Mar 24, 2025 · 6 min read
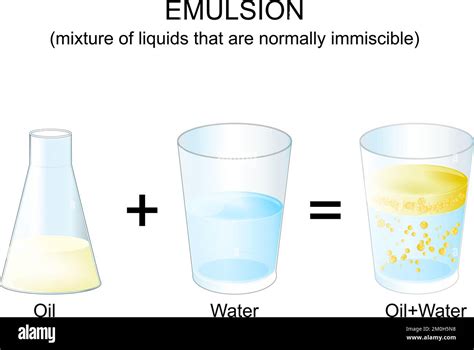
Table of Contents
What Type of Mixture is Oil and Water? Understanding Immiscibility and Emulsions
Oil and water. It's a classic example used to illustrate a fundamental concept in chemistry and everyday life: immiscibility. But what exactly is immiscibility, and what makes oil and water such a compelling illustration of this principle? This article will delve deep into the nature of oil and water mixtures, exploring the scientific reasons behind their incompatibility, examining the types of mixtures they can form under certain conditions, and discussing the practical implications of their interaction.
The Immiscibility of Oil and Water: A Tale of Polarity
The key to understanding why oil and water don't mix lies in the concept of polarity. Molecules, the building blocks of matter, possess different properties based on how their constituent atoms share electrons. Water (H₂O) is a polar molecule. This means it has a slightly positive end (the hydrogen atoms) and a slightly negative end (the oxygen atom). This uneven distribution of charge creates a dipole moment, making water a highly effective solvent for other polar substances, like sugars and salts.
Oil, on the other hand, is largely composed of nonpolar molecules. These molecules have an even distribution of charge, meaning they lack the distinct positive and negative poles found in polar molecules. Common oils, such as vegetable oil or mineral oil, are primarily made up of long hydrocarbon chains. These chains are hydrophobic, meaning they "fear" water and tend to repel it.
This difference in polarity is the fundamental reason for immiscibility. Polar molecules strongly attract each other through dipole-dipole interactions, while nonpolar molecules interact through weaker London dispersion forces. The strong attraction between water molecules prevents oil molecules from intermingling. Oil molecules would rather stick together, minimizing contact with water molecules. This is why, when oil and water are combined, they separate into distinct layers, with the less dense oil floating on top of the denser water.
Exploring Intermolecular Forces: The Driving Force Behind Separation
The behavior of oil and water is governed by the strength of intermolecular forces. Water molecules, with their strong hydrogen bonds, form a cohesive network. This network resists the intrusion of nonpolar oil molecules. The energy required to break the hydrogen bonds and force oil molecules into the water structure is significant, making the process energetically unfavorable. Consequently, the system minimizes its energy by maintaining the separation of oil and water phases.
Conversely, oil molecules, interacting through weak London dispersion forces, exhibit less cohesion. Their interaction with water molecules is even weaker, resulting in the observed separation. This disparity in intermolecular forces is the driving force behind the immiscibility of oil and water.
Types of Mixtures: Beyond Simple Separation
While oil and water generally form separate layers, it's crucial to acknowledge that under specific circumstances, they can form different types of mixtures. These include:
1. Heterogeneous Mixtures: The Classic Oil and Water Separation
The most common type of oil and water mixture is a heterogeneous mixture. This means the components remain distinct and visibly separate. You can easily see the individual oil and water layers. This is the standard scenario when you mix oil and water without any additional intervention.
2. Emulsions: Creating Stability Through Emulsifiers
An emulsion is a type of colloid where one liquid is dispersed as tiny droplets within another liquid. In the case of oil and water, this usually requires the addition of an emulsifier. Emulsifiers are substances that reduce the interfacial tension between oil and water, allowing the oil to be dispersed as stable droplets within the water (or vice-versa). The emulsifier molecules have both polar and nonpolar ends, allowing them to interact with both oil and water, effectively bridging the gap between the two immiscible phases.
Many common examples of emulsions exist, including:
- Mayonnaise: An oil-in-water emulsion where oil droplets are suspended in water, stabilized by egg yolk lecithin.
- Milk: An emulsion of fat globules in water.
- Salad dressings: Many vinaigrettes are oil-in-water emulsions, stabilized by emulsifiers like mustard.
The stability of an emulsion depends on several factors, including the type and concentration of the emulsifier, the size of the oil droplets, and the temperature. Emulsions can be either temporary or permanent, depending on the strength of the emulsifying agent.
3. Microemulsions: Enhanced Dispersion at the Nanoscale
Microemulsions are similar to emulsions but differ significantly in droplet size. In microemulsions, the oil droplets are much smaller, typically in the nanometer range. This results in a much more transparent and thermodynamically stable system compared to traditional emulsions. Microemulsions often require higher concentrations of emulsifiers and frequently incorporate co-surfactants to further enhance stability and reduce interfacial tension. Their smaller droplet size leads to improved clarity and stability, making them suitable for various applications, from pharmaceuticals to enhanced oil recovery.
Practical Applications and Implications
The interplay between oil and water, and our understanding of their mixtures, has far-reaching practical implications in various fields:
-
Food Science: The creation of emulsions is crucial in food processing, as seen in mayonnaise, salad dressings, and milk products. Controlling emulsion stability and droplet size is essential for maintaining texture and shelf life.
-
Pharmaceuticals: Many drugs are formulated as emulsions or microemulsions to enhance bioavailability and improve drug delivery. The ability to control droplet size and release profiles is critical for targeted drug delivery systems.
-
Cosmetics: Many lotions and creams are emulsions of oil and water, designed to moisturize and nourish the skin. The selection of emulsifiers and other components influences the texture and feel of the product.
-
Environmental Science: Understanding the behavior of oil and water is crucial for dealing with oil spills. Strategies for cleaning up oil spills often involve the use of dispersants, which are types of emulsifiers that break down oil into smaller droplets, allowing for easier removal.
-
Chemical Engineering: The design and operation of various chemical processes often require a detailed understanding of fluid dynamics and phase behavior. The immiscibility of oil and water plays a significant role in processes like extraction, separation, and reaction engineering.
Conclusion: A Fundamental Principle with Wide-Ranging Applications
The seemingly simple interaction of oil and water reveals a rich tapestry of scientific principles, from the fundamental nature of molecular polarity and intermolecular forces to the sophisticated manipulation of emulsions and microemulsions in various technological applications. Understanding this immiscibility is not just a matter of academic curiosity; it's a cornerstone of knowledge crucial for advancements in food science, pharmaceuticals, cosmetics, environmental science, and chemical engineering. The continuing research into the behavior of these mixtures promises even further innovative applications in the future. From the kitchen counter to the cutting edge of scientific research, the dynamic duo of oil and water continues to fascinate and inspire.
Latest Posts
Latest Posts
-
The Most Powerful Countries In 1900
Mar 29, 2025
-
Which Of The Following Gives Rise To Epithelial Tissues Only
Mar 29, 2025
-
Arrange The Following Carboxylic Acids In Order Of Acidity
Mar 29, 2025
-
What Is The Mass Of 1 Molecule Of Water
Mar 29, 2025
-
What Does Isalpha Do In Python
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Mixture Is Oil And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
