Arrange The Following Carboxylic Acids In Order Of Acidity
News Leon
Mar 29, 2025 · 6 min read
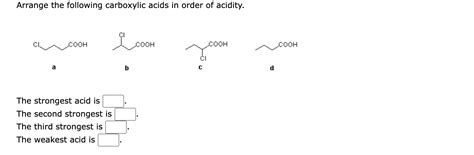
Table of Contents
Arranging Carboxylic Acids in Order of Acidity: A Comprehensive Guide
Determining the relative acidity of carboxylic acids is a fundamental concept in organic chemistry. Understanding the factors that influence acidity allows us to predict the reactivity and behavior of these important compounds. This article will delve into the intricacies of carboxylic acid acidity, exploring the key factors influencing it and providing a step-by-step approach to arranging carboxylic acids in order of increasing or decreasing acidity. We'll also examine several examples to solidify your understanding.
Understanding Carboxylic Acid Acidity
Carboxylic acids (RCOOH) are characterized by their carboxyl group (-COOH), a combination of a carbonyl group (C=O) and a hydroxyl group (-OH). The acidity of a carboxylic acid stems from the ability of the molecule to donate a proton (H⁺) from the hydroxyl group. This proton donation results in the formation of a carboxylate anion (RCOO⁻). The stability of this resulting carboxylate anion is the key determinant of the overall acidity of the carboxylic acid.
Factors Affecting Carboxylic Acid Acidity:
Several factors influence the stability of the carboxylate anion and therefore the acidity of the carboxylic acid:
-
Inductive Effects: Electron-withdrawing groups (EWGs) attached to the carbon chain near the carboxyl group stabilize the negative charge on the carboxylate anion by pulling electron density away. This stabilization increases the acidity of the carboxylic acid. Conversely, electron-donating groups (EDGs) destabilize the negative charge, decreasing acidity. The strength of the inductive effect diminishes with distance from the carboxyl group.
-
Resonance Effects: The carboxylate anion exhibits resonance stabilization. The negative charge is delocalized across both oxygen atoms, resulting in a more stable anion and increased acidity. Any substituent that enhances resonance will further increase acidity.
-
Steric Effects: Bulky substituents near the carboxyl group can hinder solvation of the carboxylate anion, reducing its stability and thus decreasing acidity. This effect is generally less pronounced than inductive and resonance effects.
-
Hybridization: The hybridization of the carbon atom bonded to the carboxyl group can influence acidity. A more electronegative carbon (e.g., sp hybridized) will withdraw electron density more effectively, increasing acidity.
Step-by-Step Approach to Arranging Carboxylic Acids by Acidity
To arrange a series of carboxylic acids in order of acidity, follow these steps:
-
Identify the substituents: Carefully examine the structure of each carboxylic acid and identify all substituents attached to the carbon chain.
-
Assess inductive effects: Determine whether each substituent is electron-withdrawing (EWG) or electron-donating (EDG). EWGs increase acidity, while EDGs decrease acidity. Consider the distance of the substituent from the carboxyl group – the closer it is, the stronger the effect.
-
Evaluate resonance effects: Determine if any substituents participate in resonance with the carboxylate anion. Substituents that enhance resonance will increase acidity.
-
Consider steric effects: While generally less significant, bulky substituents near the carboxyl group can slightly reduce acidity.
-
Arrange in order: Based on the combined effects of inductive, resonance, and steric factors, arrange the carboxylic acids in order of increasing or decreasing acidity. The carboxylic acid with the most stable carboxylate anion (due to strong EWGs, resonance, and minimal steric hindrance) will be the most acidic.
Examples: Arranging Carboxylic Acids by Acidity
Let's analyze some examples to illustrate this process:
Example 1: Arrange the following carboxylic acids in order of increasing acidity:
- Acetic acid (CH₃COOH)
- Trifluoroacetic acid (CF₃COOH)
- Chloroacetic acid (ClCH₂COOH)
Solution:
-
Substituents: Acetic acid has a methyl group (CH₃), a weak EDG. Chloroacetic acid has a chlorine atom (Cl), a strong EWG. Trifluoroacetic acid has three fluorine atoms (CF₃), very strong EWGs.
-
Inductive Effects: The fluorine atoms in trifluoroacetic acid exert a strong electron-withdrawing inductive effect, greatly stabilizing the carboxylate anion. Chlorine in chloroacetic acid also exerts an electron-withdrawing effect, but less strongly than fluorine. The methyl group in acetic acid is electron-donating, destabilizing the anion.
-
Resonance Effects: No significant resonance effects are observed in these examples.
-
Steric Effects: Steric effects are minimal in these cases.
-
Order: Therefore, the order of increasing acidity is: Acetic acid < Chloroacetic acid < Trifluoroacetic acid.
Example 2: Arrange the following carboxylic acids in order of decreasing acidity:
- Benzoic acid (C₆H₅COOH)
- p-Nitrobenzoic acid (NO₂C₆H₄COOH)
- p-Methoxybenzoic acid (CH₃OC₆H₄COOH)
Solution:
-
Substituents: Benzoic acid has a phenyl group. p-Nitrobenzoic acid has a nitro group (NO₂) at the para position. p-Methoxybenzoic acid has a methoxy group (CH₃O) at the para position.
-
Inductive and Resonance Effects: The nitro group (NO₂) is a strong EWG due to both its inductive and significant resonance effects. It strongly withdraws electron density from the ring and the carboxylate group, significantly stabilizing the anion. The methoxy group (CH₃O) is an EDG, destabilizing the anion through both inductive and resonance effects. The phenyl group in benzoic acid has a relatively weak electron-withdrawing inductive effect but its resonance contribution is relatively small compared to the nitro group.
-
Steric Effects: Steric effects are minimal.
-
Order: The order of decreasing acidity is: p-Nitrobenzoic acid > Benzoic acid > p-Methoxybenzoic acid.
Example 3: Consider the following:
- Formic acid (HCOOH)
- Acetic acid (CH₃COOH)
- Propionic acid (CH₃CH₂COOH)
Solution:
The methyl group in acetic acid is a weak electron-donating group compared to hydrogen in formic acid. The ethyl group in propionic acid is also electron-donating, but slightly weaker than the methyl group due to the increased distance from the carboxyl group. Therefore, the order of decreasing acidity is: Formic acid > Acetic acid > Propionic acid. This demonstrates the impact of alkyl group size and the weakening inductive effect with increasing distance.
Advanced Considerations: A Deeper Dive into Acidity
While the inductive and resonance effects are often the primary factors determining carboxylic acid acidity, more subtle influences can play a role in certain situations:
-
Solvent Effects: The solvent used can influence the acidity. Protic solvents, which can form hydrogen bonds, can stabilize the carboxylate anion, increasing the apparent acidity.
-
Hydrogen Bonding: The strength of hydrogen bonding between the carboxylic acid and the solvent can also affect the observed acidity.
-
Intramolecular Hydrogen Bonding: In certain cases, intramolecular hydrogen bonding can stabilize the carboxylic acid molecule, reducing its acidity.
-
Field Effects: In cases of very close proximity of specific substituents, field effects (direct electrostatic interactions) can subtly influence the acidity. These are typically less significant than inductive effects.
Conclusion: Mastering Carboxylic Acid Acidity
Understanding the factors that influence carboxylic acid acidity is crucial for predicting their reactivity and behavior. By systematically analyzing inductive effects, resonance effects, steric effects, and considering more subtle influences like solvent effects and hydrogen bonding, you can confidently arrange carboxylic acids in order of acidity. Remember to carefully examine the structure of each acid, identify all substituents, and assess their combined impact on the stability of the carboxylate anion. This comprehensive approach will provide a strong foundation for tackling more complex organic chemistry problems. Practice is key to mastering this skill, so work through numerous examples to solidify your understanding.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of A Mixture
Mar 31, 2025
-
What Is The Reciprocal Of 1 6
Mar 31, 2025
-
What Mineral Is The Hardest Known Substance In Nature
Mar 31, 2025
-
Which Organelle Is Enclosed By A Double Membrane
Mar 31, 2025
-
Compare And Contrast An Ecosystem And A Habitat
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Arrange The Following Carboxylic Acids In Order Of Acidity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
