What Type Of Energy Is Rubbing Your Hands Together
News Leon
Mar 21, 2025 · 6 min read
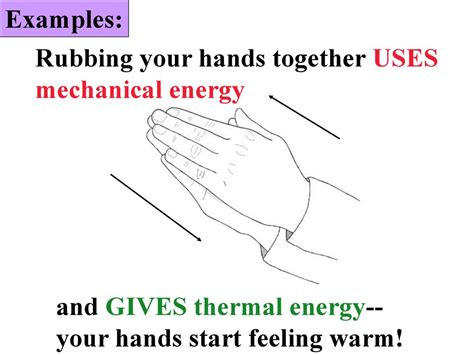
Table of Contents
What Type of Energy is Rubbing Your Hands Together? Unpacking Frictional Heat
We've all done it – rubbed our hands together on a cold day to generate warmth. But what's actually happening? This seemingly simple action is a fascinating demonstration of energy transformation, specifically the conversion of mechanical energy into thermal energy, or heat. This article will delve deep into the physics behind this process, exploring the concepts of friction, kinetic energy, and thermal energy, and providing a comprehensive understanding of the energy involved.
Understanding Friction: The Root of Heat Generation
At the heart of this heat generation lies friction. Friction is a force that opposes motion between two surfaces in contact. When you rub your hands together, the surfaces of your skin aren't perfectly smooth. They're composed of microscopic irregularities – bumps, ridges, and valleys. As you rub your hands, these irregularities interact, causing them to interlock and slide past each other.
Microscopic Interactions: The Cause of Resistance
This interlocking creates resistance to motion. The force required to overcome this resistance is the force of friction. This resistance doesn't simply vanish; it's converted into another form of energy: heat. The microscopic interactions between the surfaces create vibrations within the molecules of your skin. These vibrations represent kinetic energy at the molecular level, and the collective kinetic energy of these vibrations manifests as thermal energy – the heat you feel.
Factors Affecting Frictional Heat
Several factors influence the amount of heat generated by rubbing your hands together:
-
Pressure: Applying more pressure increases the contact area between your hands, leading to more microscopic interactions and thus greater friction and heat generation. Harder rubbing means more heat.
-
Surface Area: While seemingly counterintuitive, a larger surface area in contact can lead to increased heat generation, but only up to a point. Beyond a certain point, pressure is likely the more important factor.
-
Surface Texture: Rougher surfaces create more friction than smoother ones. Think about rubbing sandpaper versus smooth glass – the sandpaper generates significantly more heat due to its increased surface irregularities.
-
Speed: The speed at which you rub your hands together also affects heat generation. Faster rubbing means more kinetic energy is being converted into thermal energy, resulting in a more pronounced warming effect.
From Mechanical Energy to Thermal Energy: The Transformation Process
The initial energy involved in rubbing your hands together is mechanical energy. This is the energy of motion. As you move your hands, you're using the energy stored in your muscles – chemical energy converted into mechanical energy – to overcome the frictional forces.
This mechanical energy is not lost but transformed. The friction between your hands acts as a mechanism for this energy conversion. The work done against friction is dissipated as heat.
Kinetic Energy: The Molecular Perspective
At a molecular level, the mechanical energy is initially converted into kinetic energy of the molecules in your skin. The intermolecular forces and the resistance from the microscopic irregularities create vibrational motion within these molecules.
Thermal Energy: The Manifestation of Heat
The collective kinetic energy of these vibrating molecules is what we perceive as thermal energy, or heat. The increase in the average kinetic energy of the molecules translates to an increase in temperature. This explains why your hands feel warmer after rubbing them together.
Beyond Hand Rubbing: Examples of Frictional Heat in Everyday Life
The conversion of mechanical energy into thermal energy through friction isn't limited to rubbing your hands. It's a fundamental phenomenon with numerous applications and observations in everyday life:
-
Brakes: Car brakes rely on friction to slow down or stop the vehicle. The kinetic energy of the moving car is converted into heat by the friction between the brake pads and the rotors. This is why brakes can get extremely hot after prolonged use.
-
Matches: Striking a match creates friction between the match head and the striking surface. This friction generates enough heat to ignite the chemicals in the match head, resulting in a flame.
-
Drilling: Drilling into materials, whether wood or metal, generates significant heat due to the friction between the drill bit and the material. This heat can be substantial enough to require lubrication to prevent damage to both the drill bit and the material.
-
Power Generation (Historically): Early forms of power generation, like the friction wheel, utilized friction to generate heat which was then used to produce steam, which drove machinery.
The Inefficiency of Energy Conversion
It's important to note that the conversion of mechanical energy into thermal energy through friction is not perfectly efficient. Some of the initial mechanical energy is lost to other forms of energy, such as sound. When you rub your hands together, you might hear a slight squeaking sound – this is evidence of some energy being converted into sound waves. However, the majority of the energy is transformed into heat.
The Second Law of Thermodynamics and Entropy
The transformation of mechanical energy to heat through friction is consistent with the second law of thermodynamics. This law states that the total entropy of an isolated system can only increase over time, or remain constant in ideal cases where the system is in a steady state or undergoing a reversible process. In the case of rubbing your hands, the increase in entropy is reflected in the dispersal of energy as heat. The organized motion of your hands is transformed into the random motion of molecules, increasing the overall disorder or entropy of the system.
Advanced Considerations: Specific Heat Capacity and Temperature Change
The amount of heat generated by rubbing your hands together depends not just on the friction but also on the specific heat capacity of your skin. Specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. Skin has a relatively high specific heat capacity meaning that it takes a fair amount of energy to raise its temperature. Therefore, even with significant friction, the temperature increase in your hands might only be a few degrees.
Calculating the precise temperature change would require detailed knowledge of the friction force, the contact area, the duration of rubbing, the specific heat capacity of skin, and the mass of skin involved – an incredibly complex calculation beyond the scope of this article.
Conclusion: A Simple Act, a Complex Process
Rubbing your hands together to generate warmth is a simple action with profound implications in terms of energy transformation. It beautifully illustrates the fundamental principles of friction, mechanical energy, thermal energy, and the second law of thermodynamics. While the heat generated might seem insignificant, it provides a tangible demonstration of how energy can be converted from one form to another, a concept crucial to understanding numerous natural phenomena and technological applications. Understanding this seemingly simple process offers a gateway to a deeper appreciation of the physics governing our world.
Latest Posts
Latest Posts
-
How Many Atp Are Produced During Glycolysis
Mar 28, 2025
-
Pepsinogen Is Secreted By What Cells
Mar 28, 2025
-
Three Parts Of An Atp Molecule
Mar 28, 2025
-
Which Element Has Lowest Ionization Energy
Mar 28, 2025
-
Base Sequence Of Complementary Dna Strand
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Energy Is Rubbing Your Hands Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
