What Type Of Bacteria Convert Ammonia To Nitrites And Nitrates
News Leon
Mar 30, 2025 · 6 min read
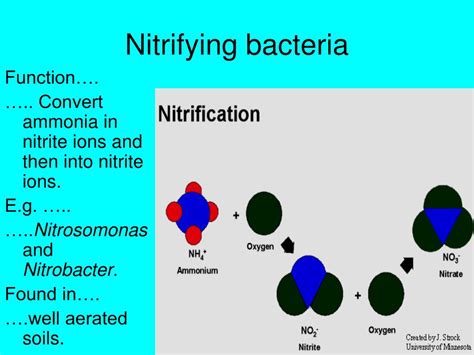
Table of Contents
What Types of Bacteria Convert Ammonia to Nitrites and Nitrates?
The nitrogen cycle is a fundamental process in all aquatic and terrestrial ecosystems, crucial for sustaining life as we know it. A critical component of this cycle is the conversion of ammonia (NH₃), a toxic byproduct of animal waste and organic decomposition, into less harmful nitrites (NO₂⁻) and then nitrates (NO₃⁻). This vital transformation is carried out by specific types of bacteria, known as nitrifying bacteria. Understanding these bacteria, their roles, and the environmental factors influencing their activity is essential for maintaining healthy ecosystems and managing wastewater treatment.
The Two-Step Process: Nitrification
Nitrification is not a single process but rather a two-step oxidation reaction carried out by two distinct groups of autotrophic bacteria:
1. Ammonia Oxidation: From Ammonia to Nitrites
The first step involves the oxidation of ammonia (NH₃) to nitrite (NO₂⁻). This process is performed by ammonia-oxidizing bacteria (AOB), belonging primarily to the genera Nitrosomonas and Nitrosococcus. These bacteria are chemoautotrophs, meaning they derive energy from the oxidation of inorganic compounds (in this case, ammonia) and use carbon dioxide as their carbon source for building biomass.
-
Nitrosomonas: This genus is widely distributed in various environments, including soil, freshwater, and marine habitats. They are particularly prevalent in aerobic conditions, requiring oxygen to carry out the oxidation process. Different species within Nitrosomonas exhibit varying levels of tolerance to environmental conditions like pH, temperature, and salinity.
-
Nitrosococcus: Another significant genus involved in ammonia oxidation, Nitrosococcus species are often found in marine environments. Similar to Nitrosomonas, they are aerobic and play a crucial role in the nitrogen cycle within marine ecosystems.
The Biochemistry of Ammonia Oxidation:
The oxidation of ammonia to nitrite is a complex enzymatic process involving several key enzymes. The most crucial enzyme is ammonia monooxygenase (AMO), which catalyzes the first step, converting ammonia to hydroxylamine (NH₂OH). This is followed by hydroxylamine oxidoreductase (HAO), which oxidizes hydroxylamine to nitrite. The overall reaction can be represented as:
NH₃ + 1.5O₂ → NO₂⁻ + H⁺ + H₂O
The energy released during this oxidation reaction is used to drive the synthesis of ATP, providing the energy needed for bacterial growth and cellular processes.
2. Nitrite Oxidation: From Nitrites to Nitrates
The second step of nitrification involves the oxidation of nitrite (NO₂⁻) to nitrate (NO₃⁻). This crucial step is performed by nitrite-oxidizing bacteria (NOB), predominantly belonging to the genus Nitrobacter. Like AOB, NOB are also chemoautotrophs, obtaining energy from the oxidation of nitrite and using carbon dioxide as their carbon source.
- Nitrobacter: This genus is ubiquitous in various environments, similar to Nitrosomonas. They are aerobic and require oxygen for the oxidation of nitrite. Different Nitrobacter species show varying tolerances to environmental factors such as pH, temperature, and nutrient availability.
The Biochemistry of Nitrite Oxidation:
The oxidation of nitrite to nitrate is catalyzed by the key enzyme nitrite oxidoreductase (NXR). This enzyme facilitates the transfer of electrons from nitrite to oxygen, generating energy in the form of ATP. The overall reaction is:
NO₂⁻ + 0.5O₂ → NO₃⁻
This energy, like in ammonia oxidation, is utilized for the growth and metabolic functions of the Nitrobacter bacteria.
Environmental Factors Influencing Nitrification
The activity of nitrifying bacteria is significantly influenced by various environmental factors, including:
-
Oxygen Availability: Both AOB and NOB are strictly aerobic, requiring sufficient dissolved oxygen for their metabolic processes. Oxygen depletion can drastically inhibit nitrification, leading to the accumulation of ammonia and nitrite, which can be toxic to aquatic life.
-
pH: Nitrification is most efficient within a relatively narrow pH range, typically between 6.5 and 8.0. Highly acidic or alkaline conditions can inhibit the activity of nitrifying bacteria, affecting the overall rate of nitrification.
-
Temperature: Temperature significantly impacts the growth and activity of nitrifying bacteria. Optimal temperatures vary depending on the specific species, but generally, nitrification rates are highest within a mesophilic temperature range (around 20-30°C). Extremely high or low temperatures can severely inhibit or even kill these bacteria.
-
Nutrient Availability: Besides oxygen and carbon dioxide, nitrifying bacteria require various nutrients, such as phosphorus, nitrogen, and trace elements, for optimal growth and activity. Nutrient deficiencies can limit the rate of nitrification.
-
Inhibitory Substances: Certain substances, such as heavy metals, organic pollutants, and some antibiotics, can inhibit the activity of nitrifying bacteria. The presence of these substances can significantly impair the nitrogen cycle and affect water quality.
Importance of Nitrifying Bacteria in Various Ecosystems
Nitrifying bacteria play essential roles in various ecosystems:
-
Wastewater Treatment: Nitrification is a cornerstone of biological wastewater treatment. Specialized reactors, such as activated sludge processes and trickling filters, utilize nitrifying bacteria to convert ammonia into less harmful nitrates, improving water quality before discharge.
-
Agriculture: In agricultural soils, nitrification is crucial for converting ammonia from fertilizers and organic matter into nitrates, the primary form of nitrogen available for plant uptake. Understanding nitrification processes is important for optimizing fertilizer application and minimizing environmental pollution from excess nitrogen.
-
Aquatic Ecosystems: In freshwater and marine environments, nitrifying bacteria play a vital role in maintaining water quality by removing ammonia, a highly toxic pollutant. The efficiency of nitrification influences the overall health and productivity of aquatic ecosystems.
-
Soil Health: Soil nitrification contributes significantly to soil fertility and overall health. The availability of nitrates for plant uptake is directly linked to the activity of nitrifying bacteria.
Recent Advances and Future Research
Research on nitrifying bacteria continues to advance, focusing on several key areas:
-
Understanding Microbial Communities: Studies are investigating the complex interactions between nitrifying bacteria and other microorganisms within various ecosystems. This includes exploring the role of other bacteria, archaea, and fungi in influencing nitrification rates and overall nitrogen cycling.
-
Developing Enhanced Nitrification Technologies: Research is focused on developing more efficient and sustainable technologies for enhancing nitrification in wastewater treatment and agriculture. This involves exploring innovative reactor designs and optimizing environmental conditions to maximize nitrification rates.
-
Exploring Novel Nitrifying Organisms: Scientists are actively searching for novel nitrifying organisms with enhanced tolerance to extreme environmental conditions, such as high salinity, high temperatures, or low oxygen levels. These organisms could have important applications in various fields, including wastewater treatment and bioremediation.
-
Genetic Engineering and Biotechnology: Genetic engineering techniques are being explored to improve the efficiency of nitrification by modifying the genes of nitrifying bacteria. This includes enhancing enzyme activity, improving tolerance to environmental stresses, and optimizing nitrogen metabolism.
Conclusion
Nitrifying bacteria, including Nitrosomonas, Nitrosococcus, and Nitrobacter, are indispensable microorganisms responsible for the vital process of nitrification, converting toxic ammonia into less harmful nitrites and nitrates. Understanding their biology, ecology, and the factors influencing their activity is crucial for maintaining healthy ecosystems, managing wastewater effectively, and optimizing agricultural practices. Ongoing research continues to unveil new insights into these fascinating microorganisms and their roles in the global nitrogen cycle, paving the way for innovative technologies and solutions for environmental management and sustainability. The intricate interplay of these bacteria within diverse environments underlines the importance of maintaining ecological balance and fostering research into their unique metabolic capabilities.
Latest Posts
Latest Posts
-
All Real Numbers Are Rational Numbers True Or False
Apr 01, 2025
-
Which Of The Following Is A Function That Money Serves
Apr 01, 2025
-
The Most Abundant Compound In Most Living Things Is
Apr 01, 2025
-
How Can We Change The Polarity Of An Electromagnet
Apr 01, 2025
-
A Mother Beats Up Her Daughter Because She Was Drunk
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bacteria Convert Ammonia To Nitrites And Nitrates . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
