What Temperature Does Water Boil On The Kelvin Scale
News Leon
Mar 19, 2025 · 5 min read
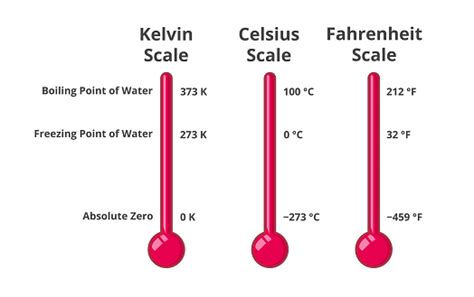
Table of Contents
What Temperature Does Water Boil on the Kelvin Scale?
The boiling point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. While we commonly express this temperature in Celsius (°C) or Fahrenheit (°F), understanding it on the Kelvin scale (K) provides a deeper insight into the fundamental nature of temperature and its implications in physics and chemistry. This article delves into the precise boiling point of water on the Kelvin scale, exploring its scientific basis, practical applications, and related concepts.
Understanding the Kelvin Scale
Before delving into the boiling point of water, let's establish a firm understanding of the Kelvin scale itself. Unlike Celsius and Fahrenheit, which are relative scales based on the freezing and boiling points of water, the Kelvin scale is an absolute temperature scale. This means its zero point, 0 K, represents absolute zero – the theoretical temperature at which all molecular motion ceases. This fundamental difference makes the Kelvin scale particularly valuable in scientific calculations and understanding thermodynamic processes.
The Relationship Between Kelvin, Celsius, and Fahrenheit
The relationship between Kelvin (K), Celsius (°C), and Fahrenheit (°F) is defined by the following conversion formulas:
- K = °C + 273.15
- °C = K - 273.15
- °F = (°C × 9/5) + 32
- °C = (°F - 32) × 5/9
These formulas allow for seamless conversion between the three scales, enabling scientists and engineers to work comfortably across different systems of measurement. Understanding these conversions is crucial for interpreting data and ensuring accurate calculations involving temperature.
The Boiling Point of Water on the Kelvin Scale
The boiling point of water at standard atmospheric pressure (1 atmosphere or 101.325 kPa) is 100°C. Using the conversion formula above, we can easily determine its equivalent on the Kelvin scale:
K = °C + 273.15 = 100°C + 273.15 = 373.15 K
Therefore, water boils at 373.15 Kelvin. This seemingly simple number has profound implications for understanding various phenomena, from steam engines to the behavior of molecules in liquids.
Factors Affecting the Boiling Point of Water
While 373.15 K is the standard boiling point, it's crucial to acknowledge that several factors can influence the actual boiling point of water:
1. Atmospheric Pressure
The most significant factor affecting the boiling point is atmospheric pressure. At higher altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Conversely, at higher pressures, such as in a pressure cooker, water boils at a higher temperature. This relationship is explained by the fact that boiling occurs when the vapor pressure of the liquid equals the surrounding atmospheric pressure. At lower pressures, less energy is required to achieve this equilibrium, resulting in a lower boiling point.
2. Impurities
The presence of dissolved substances in water, such as salts or other impurities, can also slightly elevate its boiling point. This phenomenon is known as boiling point elevation and is a colligative property, meaning it depends on the concentration of solute particles rather than their identity. The more impurities present, the higher the boiling point will be. This effect is relatively small in most everyday situations but can be significant in certain industrial processes.
3. Isotopic Composition
The isotopic composition of water molecules also plays a subtle role in the boiling point. Water molecules composed of heavier isotopes of hydrogen and oxygen (deuterium and oxygen-18) will have slightly higher boiling points compared to ordinary water. This difference is measurable but relatively insignificant for most practical applications.
Applications of the Boiling Point of Water
The knowledge of the boiling point of water, particularly on the Kelvin scale, has wide-ranging applications across various fields:
1. Chemistry and Physics
In chemistry and physics, the boiling point is a crucial property used to characterize substances, understand phase transitions, and calculate thermodynamic properties. The absolute temperature scale (Kelvin) is essential for accurate calculations involving energy, enthalpy, and entropy changes during phase transitions.
2. Engineering
Engineers rely heavily on understanding the boiling point of water in designing various systems, including steam engines, power plants, and refrigeration systems. Accurate calculations based on the Kelvin scale ensure optimal efficiency and safety in these applications. Understanding how pressure affects the boiling point is crucial for designing pressure cookers and other high-pressure systems.
3. Cooking and Food Science
In cooking, the boiling point of water determines cooking times and influences the texture of food. Understanding how altitude affects the boiling point is important for adjusting cooking times at higher elevations. Food scientists use this knowledge to develop efficient and safe cooking methods.
4. Meteorology and Climatology
In meteorology and climatology, the boiling point of water is relevant to understanding weather patterns, particularly cloud formation and precipitation processes. The temperature and pressure profiles in the atmosphere directly influence the state of water vapor.
5. Industrial Processes
Many industrial processes utilize the boiling point of water for sterilization, cleaning, and other applications. Understanding the impact of impurities and pressure on the boiling point is essential for optimizing these processes.
Advanced Concepts Related to Boiling Point
Exploring the boiling point of water leads to deeper understanding of several advanced concepts:
1. Vapor Pressure
The boiling point is intimately linked to the vapor pressure of a liquid. Vapor pressure is the pressure exerted by the vapor phase of a substance in equilibrium with its liquid phase. Boiling occurs when the vapor pressure equals the external pressure.
2. Critical Point
Every substance has a critical point, the temperature and pressure above which the distinction between liquid and gas phases disappears. Understanding the critical point of water is essential for high-pressure applications and advanced thermodynamic studies.
3. Phase Diagrams
Phase diagrams graphically represent the relationship between temperature, pressure, and the phases of a substance. The boiling point is prominently featured on these diagrams, illustrating the conditions under which water exists as a liquid, vapor, or a mixture of both.
Conclusion
The boiling point of water at 373.15 K is not merely a numerical value; it represents a fundamental physical constant with far-reaching implications across numerous scientific and technological fields. Understanding the factors influencing the boiling point, its relationship to other thermodynamic properties, and its various applications is crucial for anyone involved in scientific research, engineering, or any field where temperature plays a critical role. The Kelvin scale's absolute nature provides a robust and consistent framework for these crucial calculations and interpretations, making it invaluable for a precise understanding of this seemingly simple phenomenon. Further exploration into these concepts will undoubtedly continue to unveil the rich complexity and importance of the boiling point of water in the broader context of scientific discovery and technological advancement.
Latest Posts
Latest Posts
-
How Many Micrograms In A Kilogram
Mar 19, 2025
-
A Food Chain Starts With An
Mar 19, 2025
-
Which Gametes Can A Rryy Plant Produce
Mar 19, 2025
-
How Many Hearts Are In A Deck Of 52 Cards
Mar 19, 2025
-
Sun Distance From Earth In Light Years
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Temperature Does Water Boil On The Kelvin Scale . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
