What State Of Matter Is Compressible
News Leon
Mar 22, 2025 · 5 min read
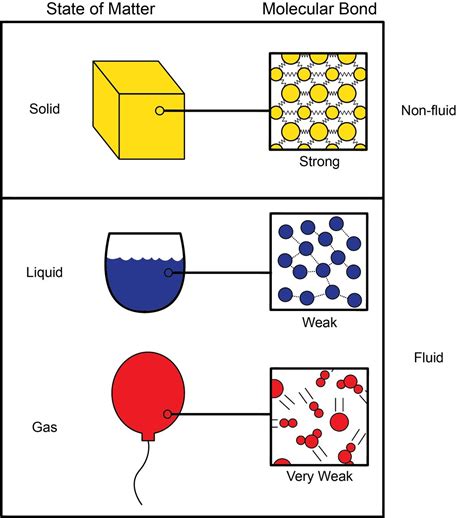
Table of Contents
What State of Matter is Compressible? Understanding the Relationship Between Matter, Pressure, and Volume
The question of which state of matter is compressible isn't a simple yes or no answer. While some states are significantly more compressible than others, the degree of compressibility depends on various factors, including the intermolecular forces, temperature, and the applied pressure. Let's delve into the fascinating world of matter and explore the compressibility of solids, liquids, and gases.
The Nature of Compressibility
Compressibility refers to the ability of a substance to reduce its volume under the application of external pressure. Essentially, it's how easily a substance can be squeezed into a smaller space. This ability is directly related to the distance between the constituent particles (atoms, ions, or molecules) of the substance. The closer these particles are, the less compressible the substance will be.
Solids: The Least Compressible
Solids are generally the least compressible of the three primary states of matter. This is because the constituent particles in a solid are tightly packed together in a fixed arrangement, held firmly in place by strong intermolecular forces. There's very little free space between the particles, limiting the extent to which they can be pushed closer together.
Exceptions in Solid Compressibility:
While generally incompressible, some solids exhibit a degree of compressibility under extremely high pressures. For instance, in certain geological processes deep within the Earth, immense pressures can cause rocks and minerals to compress slightly. This is also exploited in industrial processes like the formation of high-density materials.
-
Porous Solids: Materials like wood or pumice, which contain significant voids or pores within their structure, exhibit greater compressibility than dense solids like metals. The compression primarily occurs by reducing the size of these internal spaces.
-
Amorphous Solids: Amorphous solids, like glass, lack the ordered crystalline structure of other solids. This somewhat less organized structure may allow for slightly greater compressibility compared to their crystalline counterparts.
Liquids: Moderately Compressible
Liquids are significantly more compressible than solids, but still much less so than gases. The particles in a liquid are closer together than in a gas but further apart than in a solid, and they are not fixed in a rigid structure. Therefore, there is some space between the particles that can be reduced under pressure, resulting in a decrease in volume. However, the strong intermolecular forces still prevent substantial compression.
Factors Affecting Liquid Compressibility:
-
Temperature: Generally, the compressibility of liquids decreases with increasing temperature. Higher temperatures increase the kinetic energy of the particles, making them resist compression more effectively.
-
Pressure: The compressibility of liquids is non-linear. At low pressures, the change in volume is proportional to the applied pressure, but at high pressures, the compressibility decreases significantly.
-
Type of Liquid: Different liquids possess different levels of compressibility due to variations in their intermolecular forces and molecular structures.
Gases: Highly Compressible
Gases are by far the most compressible state of matter. This is because the particles in a gas are widely dispersed, with significant empty space between them. The weak intermolecular forces allow the particles to be easily pushed closer together under pressure, resulting in a substantial reduction in volume. This is the basis for many practical applications, from pneumatic systems to the storage of natural gas.
Understanding Gas Compressibility:
-
Ideal Gas Law: The behavior of gases, especially at relatively low pressures and high temperatures, is often modeled using the ideal gas law: PV = nRT. This equation demonstrates the inverse relationship between pressure (P) and volume (V) – as pressure increases, volume decreases, and vice versa. This illustrates the significant compressibility of gases.
-
Real Gases: While the ideal gas law provides a useful approximation, real gases deviate from this behavior, particularly at high pressures or low temperatures. At high pressures, the intermolecular forces become more significant, reducing the compressibility compared to the ideal gas model. At low temperatures, the kinetic energy of the particles decreases, making them more susceptible to intermolecular forces and affecting compressibility.
Beyond Solids, Liquids, and Gases: Plasma and Bose-Einstein Condensates
The discussion above focuses primarily on the three common states of matter. However, there are other states, each with its unique compressibility characteristics:
-
Plasma: Plasma, often considered the fourth state of matter, is an ionized gas. While it shares some similarities with gases in terms of compressibility, the presence of charged particles introduces complexities in its behavior under pressure. Compressibility in plasma depends on factors such as temperature, density, and magnetic fields.
-
Bose-Einstein Condensates: At extremely low temperatures, certain atoms can condense into a Bose-Einstein condensate, a state where a large number of atoms occupy the same quantum state. The compressibility of a Bose-Einstein condensate is a complex topic involving quantum mechanics and is often described using concepts from quantum field theory.
Compressibility in Everyday Life and Industrial Applications
The compressibility of different states of matter plays a crucial role in numerous applications:
-
Pneumatic Systems: Air compressors utilize the high compressibility of gases to generate pressurized air used in various tools and machinery.
-
Hydraulic Systems: While liquids are less compressible than gases, their slight compressibility is still important in hydraulic systems, influencing the response time and efficiency of the system.
-
Packaging: The compressibility of materials is considered in packaging design, ensuring products are protected during transport and storage.
-
Geophysics: The compressibility of rocks and minerals under immense pressure influences geological processes such as plate tectonics and earthquake formation.
-
Material Science: Understanding compressibility is crucial in material science for the design of new materials with specific properties, such as high-pressure seals or shock absorbers.
Conclusion: A Matter of Degree
The compressibility of matter is not an absolute property but rather a spectrum. Gases are highly compressible, liquids are moderately compressible, and solids are generally the least compressible. However, even solids can be compressed under extreme pressures, and the compressibility of each state can be influenced by factors like temperature, pressure, and the specific properties of the material. Understanding the compressibility of different states of matter is crucial in various fields, from engineering and technology to geology and materials science. This knowledge helps us design and utilize materials and systems effectively, harnessing the properties of different states of matter to suit our needs. The ongoing research in materials science and physics continues to deepen our understanding of compressibility under diverse conditions, leading to innovations across numerous scientific and engineering disciplines.
Latest Posts
Latest Posts
-
Rutherford Conclusion From Gold Foil Experiment
Mar 22, 2025
-
What Two Monosaccharides Make Up Lactose
Mar 22, 2025
-
What Is The Opposite Of Obsolete
Mar 22, 2025
-
Which Of The Following Is Not Associated With Viruses
Mar 22, 2025
-
Complete The Following Table Regarding Acids And Bases
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about What State Of Matter Is Compressible . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
