. Complete The Following Table Regarding Acids And Bases.
News Leon
Mar 22, 2025 · 6 min read
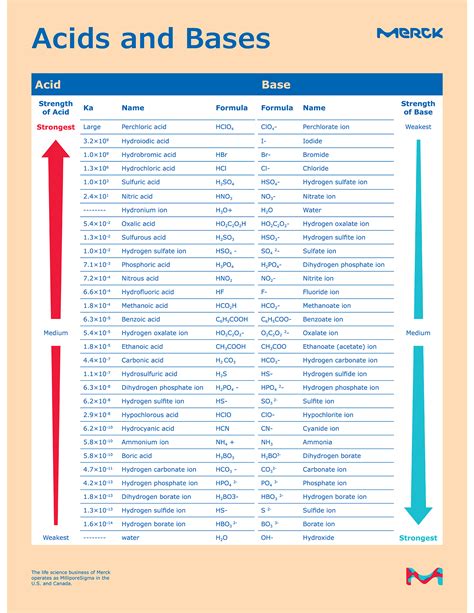
Table of Contents
Understanding Acids and Bases: A Comprehensive Guide
Acids and bases are fundamental concepts in chemistry, impacting numerous aspects of our daily lives, from the food we eat to the medications we take. Understanding their properties, reactions, and classifications is crucial for anyone studying chemistry or related fields. This comprehensive guide will delve into the intricacies of acids and bases, exploring various definitions, properties, and examples. We'll also clarify common misconceptions and provide a detailed explanation to complete the table you requested.
Defining Acids and Bases: A Multifaceted Approach
Several theories attempt to define acids and bases, each offering a unique perspective on their behavior. The most common are the Arrhenius, Brønsted-Lowry, and Lewis theories.
1. Arrhenius Theory: This is the simplest definition, stating that an acid is a substance that produces hydrogen ions (H⁺) when dissolved in water, while a base produces hydroxide ions (OH⁻) in water. For example, hydrochloric acid (HCl) dissociates into H⁺ and Cl⁻ ions in water, acting as an acid, whereas sodium hydroxide (NaOH) dissociates into Na⁺ and OH⁻ ions, behaving as a base.
Limitations: The Arrhenius theory is limited because it only applies to aqueous solutions (solutions in water). Many acid-base reactions occur in non-aqueous solvents, where this definition doesn't apply.
2. Brønsted-Lowry Theory: This theory expands upon the Arrhenius theory by defining an acid as a proton donor and a base as a proton acceptor. A proton is simply a hydrogen ion (H⁺). This definition allows for acid-base reactions in non-aqueous solutions. For instance, in the reaction between ammonia (NH₃) and hydrogen chloride (HCl), HCl donates a proton to NH₃, making HCl the Brønsted-Lowry acid and NH₃ the Brønsted-Lowry base.
Advantages: The Brønsted-Lowry theory encompasses a broader range of reactions than the Arrhenius theory, including those occurring in non-aqueous solvents. It also introduces the concept of conjugate acid-base pairs. When an acid donates a proton, the remaining species is its conjugate base. Similarly, when a base accepts a proton, the resulting species is its conjugate acid.
3. Lewis Theory: The Lewis theory provides the most general definition of acids and bases. A Lewis acid is defined as an electron pair acceptor, while a Lewis base is an electron pair donor. This definition encompasses many reactions that aren't considered acid-base reactions according to the Arrhenius or Brønsted-Lowry theories. For example, boron trifluoride (BF₃) acts as a Lewis acid because it can accept an electron pair, while ammonia (NH₃) acts as a Lewis base because it can donate an electron pair.
Advantages: The Lewis theory is the most inclusive, expanding the scope of acid-base chemistry beyond proton transfer. It explains reactions that involve coordinate covalent bonds, where one atom provides both electrons in the bond.
Properties of Acids and Bases
Acids and bases exhibit distinct properties that allow for their identification and characterization.
Properties of Acids:
- Sour taste: This is a classic characteristic, though it should never be tested directly due to potential harm.
- React with metals: Acids react with active metals (like zinc and magnesium) to produce hydrogen gas.
- Change the color of indicators: Indicators like litmus paper turn red in acidic solutions.
- Lower pH: Acids have a pH value less than 7.
- Conduct electricity: Acids in solution conduct electricity because they dissociate into ions.
Properties of Bases:
- Bitter taste: Similar to acids, this should never be tested directly.
- Slippery or soapy feel: Many bases feel slippery when touched.
- Change the color of indicators: Bases turn litmus paper blue.
- Higher pH: Bases have a pH value greater than 7.
- Conduct electricity: Bases in solution conduct electricity due to ion dissociation.
The pH Scale
The pH scale is a logarithmic scale used to measure the acidity or basicity of a solution. It ranges from 0 to 14, with 7 representing a neutral solution (neither acidic nor basic). A pH less than 7 indicates acidity, while a pH greater than 7 indicates basicity. Each whole number change on the pH scale represents a tenfold change in hydrogen ion concentration. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
Examples of Acids and Bases
Strong Acids: These acids completely dissociate into ions in water. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and nitric acid (HNO₃).
Weak Acids: These acids only partially dissociate in water. Examples include acetic acid (CH₃COOH) found in vinegar and carbonic acid (H₂CO₃) found in carbonated drinks.
Strong Bases: These bases completely dissociate into ions in water. Examples include sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Weak Bases: These bases only partially dissociate in water. An example is ammonia (NH₃).
Completing the Table: Acids and Bases
Now, let's address your request to complete a table regarding acids and bases. Since you did not provide a table structure, I will create one encompassing key properties and examples. You can adapt this table to your specific needs.
| Feature | Acid | Base |
|---|---|---|
| Definition (Arrhenius) | Produces H⁺ ions in water | Produces OH⁻ ions in water |
| Definition (Brønsted-Lowry) | Proton (H⁺) donor | Proton (H⁺) acceptor |
| Definition (Lewis) | Electron pair acceptor | Electron pair donor |
| Taste | Sour | Bitter |
| Feel | Typically none, can be corrosive | Slippery, soapy |
| pH | Less than 7 | Greater than 7 |
| Effect on Litmus Paper | Turns red | Turns blue |
| Reaction with Metals | Reacts with active metals (H₂ gas) | Generally does not react with metals |
| Electrical Conductivity | Conducts electricity in solution | Conducts electricity in solution |
| Examples (Strong) | HCl, H₂SO₄, HNO₃ | NaOH, KOH |
| Examples (Weak) | CH₃COOH, H₂CO₃, H₃PO₄ | NH₃, Mg(OH)₂, Al(OH)₃ |
Neutralization Reactions
Acids and bases react with each other in a process called neutralization. This reaction typically produces water and a salt. For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) produces water (H₂O) and sodium chloride (NaCl), common table salt:
HCl(aq) + NaOH(aq) → H₂O(l) + NaCl(aq)
Applications of Acids and Bases
Acids and bases play crucial roles in various applications:
- Industrial Processes: Acids are used in manufacturing fertilizers, plastics, and detergents. Bases are used in paper production, soap making, and the refining of metals.
- Food and Beverages: Acids like citric acid and acetic acid are used as preservatives and flavor enhancers. Bases are used in baking and food processing.
- Medicine: Many medications contain acids or bases. Antacids, for example, use bases to neutralize stomach acid.
- Cleaning Products: Many household cleaners contain acids or bases to remove dirt, grease, and stains.
Safety Precautions
Acids and bases can be corrosive and harmful. Always handle them with care, wearing appropriate safety equipment like gloves and eye protection. Follow instructions carefully and never mix acids and bases without understanding the potential consequences. In case of accidental contact, immediately rinse the affected area with plenty of water and seek medical attention if necessary.
Conclusion
Understanding the properties, reactions, and applications of acids and bases is essential in chemistry and numerous related fields. This comprehensive guide, including the detailed table, should provide a solid foundation for further exploration of this fascinating topic. Remember to always prioritize safety when working with acids and bases. Further research into specific acid-base reactions and applications will deepen your understanding and allow you to apply this knowledge effectively.
Latest Posts
Latest Posts
-
What Is The Distance Between Points M And N Meters
Mar 23, 2025
-
Find The Mean Of First Nine Prime Numbers
Mar 23, 2025
-
150 Is 96 Of What Number
Mar 23, 2025
-
Oxidation State Of Sulfur In H2so4
Mar 23, 2025
-
How Many Heart Chambers Do Fish Have
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about . Complete The Following Table Regarding Acids And Bases. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
